Comparison of Dual Monoclonal Antibody Therapies for COVID-19 Evolution: A Multicentric Retrospective Study
- PMID: 39459877
- PMCID: PMC11512400
- DOI: 10.3390/v16101542
Comparison of Dual Monoclonal Antibody Therapies for COVID-19 Evolution: A Multicentric Retrospective Study
Abstract
Background: Neutralizing antibodies targeting the SARS-CoV-2 Spike protein reduce COVID-19-related risk of hospitalization, particularly in high-risk individuals. The COCOPREV-R study aimed to evaluate and compare clinical outcomes in high-risk SARS-CoV-2 patients treated with dual monoclonal antibody therapies and to identify associated virological factors.
Methods: The COCOPREV-R study retrospectively collected real-world data from high-risk patients receiving Bamlanivimab/Etesevimab or Casirivimab/Imdevimab dual monoclonal antibody therapies (22 February 2021 to 15 June 2021).
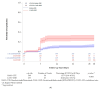
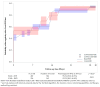
Results: The study included 1004 patients with COVID-19, of whom 691 received Bamlanivimab/Etesevimab and 313 received Casirivimab/Imdevimab. The alpha variant represented 90.1% of those for whom data were available. The risk of hospitalization within 30 days was lower with Bamlanivimab/Etesevimab (12.7%, CI 95% [9.9-16.3%]) compared to Casirivimab/Imdevimab (28.4%, CI 95% [22.7-35.1%) (p < 0.001). The 30-day mortality rates were comparable between both groups (p = 0.982). Analysis of SARS-CoV-2 PCR negativity showed no difference between the two treatment groups (95.2% [93.0-96.9%] and 93.5% [89.1-96.6%] until day 30, p = 0.851 for Bamlanivimab/Etesevimab and Casirivimab/Imdevimab, respectively). Among persistently positive samples with available sequencing results (n = 43), Spike protein changes occurred only in Bamlanivimab/Etesevimab (42.9%) vs. Casirivimab/Imdevimab (0.0%) groups. Q493R (25.0%) and E484K (12.5%) were the most common mutations selected by Bamlanivimab/Etesevimab in follow-up samples. Other factors (immunodepression, comorbidities, and age) did not appear to be associated with the occurrence of Spike protein mutations.
Conclusions: A higher rate of hospitalization was seen with Casirivimab/Imdevimab (RONAPREVE®) in comparison with Bamlanivimab/Etesevimab treatment, but with the emergence of Spike mutations only in the Bamlanivimab/Etesevimab group.
Keywords: COVID-19; SARS-CoV-2; immune escape mutation; monoclonal antibody therapy; spike gene.
Conflict of interest statement
The authors declare that they have no competing interests with the current work.
Figures


References
-
- Dougan M., Azizad M., Mocherla B., Gottlieb R.L., Chen P., Hebert C., Perry R., Boscia J., Heller B., Morris J., et al. A Randomized, Placebo-Controlled Clinical Trial of Bamlanivimab and Etesevimab Together in High-Risk Ambulatory Patients With COVID-19 and Validation of the Prognostic Value of Persistently High Viral Load. Clin. Infect. Dis. 2022;75:e440–e449. doi: 10.1093/cid/ciab912. - DOI - PMC - PubMed
-
- Abani O., Abani O., Abbas A., Abbas A., Abbas F., Abbas F., Abbas M., Abbas M., Abbasi S., Abbasi S., et al. Casirivimab and Imdevimab in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. Lancet. 2022;399:665–676. doi: 10.1016/S0140-6736(22)00163-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

