Protoporphyrin IX-Dependent Antiviral Effects of 5-Aminolevulinic Acid against Feline Coronavirus Type II
- PMID: 39459928
- PMCID: PMC11512371
- DOI: 10.3390/v16101595
Protoporphyrin IX-Dependent Antiviral Effects of 5-Aminolevulinic Acid against Feline Coronavirus Type II
Abstract
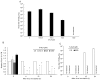
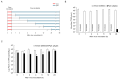
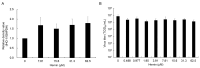
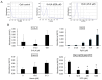
5-Aminolevulinic acid (5-ALA), a non-proteinogenic amino acid, is an intermediate in the biosynthesis of heme and exerts antiviral effects against feline coronavirus (FCoV); however, the underlying mechanisms remain unclear. In the biosynthesis of heme, 5-ALA is condensed and converted to protoporphyrin IX (PpIX), which is then transformed into heme by the insertion of ferrous iron. Previous research has suggested that the metabolites generated during heme biosynthesis contribute to the antiviral effects of 5-ALA. Therefore, the present study investigated the in vitro mechanisms responsible for the antiviral effects of 5-ALA. The results obtained revealed that 5-ALA and PpIX both effectively reduced the viral titer in the supernatant of FCoV-infected fcwf-4 cells. Moreover, PpIX exerted virucidal effects against FCoV. We also confirmed that 5-ALA increased PpIX levels in cells. While hemin induced heme oxygenase-1 gene expression, it did not reduce the viral titer in the supernatant. Sodium ferrous citrate decreased PpIX levels and suppressed the antiviral effects of 5-ALA. Collectively, these results suggest that the antiviral effects of 5-ALA against FCoV are dependent on PpIX.
Keywords: 5-aminolevulinic acid; feline coronavirus; protoporphyrin IX.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Taxon Details|ICTV. [(accessed on 24 July 2024)]. Available online: https://ictv.global/taxonomy/taxondetails?taxnode_id=202301849&taxon_nam....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

