A Novel Monoclonal Antibody Against a Modified Vaccinia Ankara (MVA) Envelope Protein as a Tool for MVA Virus Titration by Flow Cytometry
- PMID: 39459960
- PMCID: PMC11512277
- DOI: 10.3390/v16101628
A Novel Monoclonal Antibody Against a Modified Vaccinia Ankara (MVA) Envelope Protein as a Tool for MVA Virus Titration by Flow Cytometry
Abstract
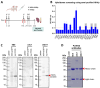
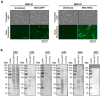
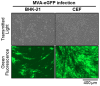
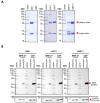
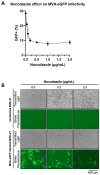
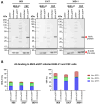
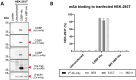
Modified vaccinia Ankara (MVA) virus is a widely used vaccine platform, making accurate titration essential for vaccination studies. However, the current plaque forming unit (PFU) assay, the standard for MVA titration, is prone to observer bias and other limitations that affect accuracy and precision. To address these challenges, we developed a new flow cytometry-based quantification method using a highly specific monoclonal antibody (mAb) for the detection of MVA-infected cells, as a more accurate titration assay. Through previous work, we serendipitously identified three MVA-specific hybridoma antibody clones, which we characterized through ELISA, immunoblot, and flow cytometry, confirming their specificity for MVA. Sequencing confirmed that each antibody was monoclonal, and mass spectrometry results revealed that all mAbs target the MVA cell surface binding protein (CSBP, MVA105L). We next optimized the titration protocol using the most effective mAb, 33C7 by refining culture conditions and staining protocols to enhance sensitivity and minimize background. Our optimized method demonstrated superior sensitivity, reliability, and reduced processing time when compared with the traditional PFU assay, establishing it as a more accurate and efficient approach for MVA titration.
Keywords: D8L; MVA105L; OPG105; TCID50; cell surface binding protein; flow cytometry; modified vaccinia Ankara; monoclonal antibody; plaque-forming unit assay; virus titration.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures







References
-
- Puri A., Pollard A.J., Schmidt-Mutter C., Lainé F., PrayGod G., Kibuuka H., Barry H., Nicolas J.F., Lelièvre J.D., Sirima S.B., et al. Long-Term Clinical Safety of the Ad26.ZEBOV and MVA-BN-Filo Ebola Vaccines: A Prospective, Multi-Country, Observational Study. Vaccines. 2024;12:210. doi: 10.3390/vaccines12020210. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

