A New Licensed Quadrivalent Antileptospiral Canine Vaccine Prevents Mortality, Clinical Signs, Infection, Bacterial Excretion, Renal Carriage and Renal Lesions Caused by Leptospira Australis Experimental Challenge
- PMID: 39460272
- PMCID: PMC11511386
- DOI: 10.3390/vaccines12101104
A New Licensed Quadrivalent Antileptospiral Canine Vaccine Prevents Mortality, Clinical Signs, Infection, Bacterial Excretion, Renal Carriage and Renal Lesions Caused by Leptospira Australis Experimental Challenge
Abstract
Background: L. Australis is one of the most prevalent Leptospira strains infecting dogs, leading, in natural conditions, to severe life-threatening cases.
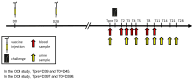
Objective: The objective was to evaluate the onset and duration of immunity (OOI and DOI) induced by a new licensed quadrivalent antileptospiral vaccine (EURICAN® L4) including four Leptospira components (Canicola, Icterohaemorrhagiae, Grippotyphosa and Australis) against L. Australis. To this end, a severe L. Australis challenge model was developed, using a canine strain recently isolated from the field.
Material and methods: Seven- to ten-week-old puppies received two doses of the vaccine four weeks apart and were challenged with an L. Australis isolate two weeks (OOI) and 12 months (DOI) later. Mortality, clinical signs, leptospiremia, leptospiruria, renal carriage, and renal lesions were assessed after challenge.
Results: The challenge induced multiple severe clinical signs in controls, leading to the death or euthanasia of 83% of puppies and 57% of adults. In controls, leptospiremia was detected in all dogs, leptospiruria in 67% of puppies and 86% of adults, kidneys tested positive for Leptospira in 83% of puppies and 71% of adults, and kidney lesions were observed in 100% of puppies and 86% of adults. In addition, thrombocytopenia associated with increased concentrations of urea, creatinine, and aspartate aminotransferase was recorded in controls displaying severe clinical signs. In both OOI and DOI studies, none of the vaccinates had clinical signs, no Leptospira was detected in blood, urine, and kidney samples, and no kidney lesions were observed in vaccinates. No significant changes in hematological and biochemical parameters in vaccinates were recorded.
Conclusion: EURICAN® L4 was shown to induce quick and long-lasting protection against a severe L. Australis infectious challenge, preventing mortality, clinical signs, infection, bacterial excretion, renal lesions, and renal carriage.
Keywords: Australis; Leptospira; challenge; dog; vaccine.
Conflict of interest statement
The authors of this paper are employees of Boehringer Ingelheim, the manufacturer of EURICAN®L4. The contribution of Anne-Laure GUIOT to the publication was funded by Boehringer Ingelheim.
Figures



References
LinkOut - more resources
Full Text Sources

