Immunogenicity and Protectivity of Sputnik V Vaccine in hACE2-Transgenic Mice against Homologous and Heterologous SARS-CoV-2 Lineages Including Far-Distanced Omicron BA.5
- PMID: 39460319
- PMCID: PMC11512357
- DOI: 10.3390/vaccines12101152
Immunogenicity and Protectivity of Sputnik V Vaccine in hACE2-Transgenic Mice against Homologous and Heterologous SARS-CoV-2 Lineages Including Far-Distanced Omicron BA.5
Abstract
Background: The SARS-CoV-2 virus continuously acquires mutations, leading to the emergence of new variants. Notably, the effectiveness of global vaccination efforts has significantly declined with the rise and spread of the B.1.1.529 (Omicron) variant.
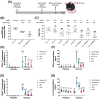
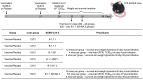
Methods: The study used virological, immunological and histological research methods, as well as methods of working with laboratory animals. In this study, we evaluated the Gam-COVID-Vac (Sputnik V), an adenoviral vaccine developed by the N.F. Gamaleya National Research Center for Epidemiology and Microbiology, and conducted experiments on hemizygous K18-ACE2-transgenic F1 mice. The variants studied included B.1.1.1, B.1.1.7, B.1.351, B.1.1.28/P.1, B.1.617.2, and B.1.1.529 BA.5.
Results: Our findings demonstrate that the Sputnik V vaccine elicits a robust humoral and cellular immune response, effectively protecting vaccinated animals from challenges posed by various SARS-CoV-2 variants. However, we observed a notable reduction in vaccine efficacy against the B.1.1.529 (Omicron BA.5) variant.
Conclusions: Our results indicate that ongoing monitoring of emerging mutations is crucial to assess vaccine efficacy against new SARS-CoV-2 variants to identify those with pandemic potential. If protective efficacy declines, it will be imperative to develop new vaccines tailored to current variants of the virus.
Keywords: COVID-19 vaccine; cross-protection; hACE2-transgenic mice; immunogenicity; vector vaccine.
Conflict of interest statement
I.V.D., A.I.T., D.M.G., A.G.B., F.M.I., A.S.D., A.S.E., O.P., T.A.O., A.S.S., O.V.Z., D.V.S., D.Y.L., B.S.N. and G.A.L. report patents for a Sputnik V pharmaceutical agent, and its method of use to prevent COVID-19. All other authors declare no competing interests.
Figures



References
-
- World Health Organization COVID-19 Public Health Emergency of International Concern (PHEIC) Global Research and Innovation Forum. [(accessed on 3 October 2024)]. Available online: https://www.who.int/publications/m/item/covid-19-public-health-emergency....
-
- World Health Organization WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. [(accessed on 3 October 2024)]. Available online: https://www.who.int/director-general/speeches/detail/who-director-genera....
-
- World Health Organization WHO COVID-19 Dashboard. [(accessed on 3 October 2024)]. Available online: https://data.who.int/dashboards/covid19/cases?n=c.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

