Modelling the Relative Vaccine Efficacy of ARCT-154, a Self-Amplifying mRNA COVID-19 Vaccine, versus BNT162b2 Using Immunogenicity Data
- PMID: 39460327
- PMCID: PMC11511100
- DOI: 10.3390/vaccines12101161
Modelling the Relative Vaccine Efficacy of ARCT-154, a Self-Amplifying mRNA COVID-19 Vaccine, versus BNT162b2 Using Immunogenicity Data
Abstract
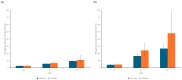
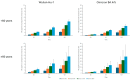
Background: Self-amplifying mRNA vaccines have the potential to increase the magnitude and duration of protection against COVID-19 by boosting neutralizing antibody titers and cellular responses. Methods: In this study, we used the immunogenicity data from a phase 3 randomized trial comparing the immunogenicity of ARCT-154, a self-amplifying mRNA COVID-19 vaccine, with BNT162b2 mRNA COVID-19 vaccine to estimate the relative vaccine efficacy (rVE) of the two vaccines over time in younger (<60 years) and older (≥60 years) adults. Results: By day 181 post-vaccination, the rVE against symptomatic and severe Wuhan-Hu-1 disease was 9.2-11.0% and 1.2-1.5%, respectively, across age groups whereas the rVE against symptomatic and severe Omicron BA.4/5 disease was 26.8-48.0% and 5.2-9.3%, respectively, across age groups. Sensitivity analysis showed that varying the threshold titer for 50% protection against severe disease up to 10% of convalescent sera revealed incremental benefits of ARCT-154 over BNT162b2, with an rVE of up to 28.0% against Omicron BA.4/5 in adults aged ≥60 year. Conclusions: Overall, the results of this study indicate that ARCT-154 elicits broader and more durable immunogenicity against SARS-CoV-2, translating to enhanced disease protection, particularly for older adults against Omicron BA.4/5.
Keywords: ARCT-154; BNT162b2; efficacy; older adults; self-amplifying mRNA vaccine.
Conflict of interest statement
Van Hung Nguyen and Jean Marie Pivette: Employees of VHN Consulting, which received funding from CSL Seqirus for the analysis described in the manuscript and for manuscript development. Pascal Crépey received funding from VHN Consulting for the analysis described in the manuscript. Ethan Settembre, Sankarasubramanian Rajaram, John Youhanna, Aimee Ferraro, Cheng Chang, Josephine van Boxmeer, and Joaquin F. Mould-Quevedo: Employees of CSL Seqirus.
Figures
References
-
- Centers for Disease Control and Prevention Trends in United States COVID-19 Hospitalizations, Deaths, Emergency Department (ED) Visits, and Test Positivity by Geographic Area. Centers for Disease Control and Prevention. [(accessed on 15 April 2024)]; Available online: https://covid.cdc.gov/covid-data-tracker.
-
- UKHSA UKHSA Data Dashboard: COVID-19. [(accessed on 25 June 2024)]; Available online: https://ukhsa-dashboard.data.gov.uk/topics/covid-19#healthcare.
-
- Chakraborty C., Bhattacharya M., Dhama K. SARS-CoV-2 Vaccines, Vaccine Development Technologies, and Significant Efforts in Vaccine Development during the Pandemic: The Lessons Learned Might Help to Fight against the Next Pandemic. Vaccines. 2023;11:682. doi: 10.3390/vaccines11030682. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention Interim Clinical Considerations for Use of COVID-19 Vaccines in the United States. [(accessed on 12 February 2024)]; Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-co....
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

