Impact of the adenosine receptor A2BR expressed on myeloid cells on immune regulation during pregnancy
- PMID: 39460389
- PMCID: PMC11628929
- DOI: 10.1002/eji.202451149
Impact of the adenosine receptor A2BR expressed on myeloid cells on immune regulation during pregnancy
Abstract
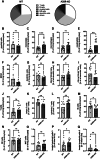
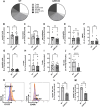
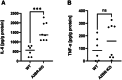
During pregnancy, the maternal immune system must carefully balance protection against pathogens with tolerance toward the semiallogeneic fetus. Dysfunctions of the immune system can lead to severe complications such as preeclampsia, fetal growth restriction, or pregnancy loss. Adenosine plays a role in physiological processes and plasma-level increase during pregnancy. The adenosine receptor A2B (A2BR), which is expressed on both, immune and nonimmune cells, is activated by high adenosine concentrations, achieved during pregnancy. We investigated the impact of A2BR expressed on myeloid cells on immune regulation during pregnancy using a mouse model with myeloid deficiency for A2BR. We demonstrate systemic changes in myeloid and lymphoid cell populations during pregnancy in A2BR-KO (Adora2B923f/f-LysMCre) mice with increased monocytes, neutrophils, and T cells but decreased B cells as well as altered T-cell subpopulations with decreased Th1 cells and Tregs and increased Th17 cells. Lack of A2BR on myeloid cells caused an increased systemic expression of IL-6 but decreased systemic accumulation and function of MDSC and reduced numbers of uterine natural killer cells. The pregnancy outcome was only marginally affected. Our results demonstrate that A2BR on myeloid cells plays a role in immune regulation during pregnancy, but the clinical impact on pregnancy remains unclear.
Keywords: Adenosine receptor; Immune regulation; Myeloid A2BR knockout; Pregnancy.
© 2024 The Author(s). European Journal of Immunology published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Rai, R. and Regan, L. , Recurrent miscarriage. Lancet 2006. 368: 601–611. - PubMed
-
- Zenclussen, A. C. , Gentile, T. , Margni, R. , Kortebani, G. and Mazzolli, A. , Asymmetric antibodies and pregnancy. Am. J. Reprod. Immunol. 2001. 45: 289–294. - PubMed
-
- Clark, D. A. , Coulam, C. B. , Daya, S. and Chaouat, G. , Unexplained sporadic and recurrent miscarrage in the new millennium: a critical analysis of immune mechanisms and treatments. Hum. Reprod. Update 2001. 7: 501–511. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

