MBNL deficiency in motor neurons disrupts neuromuscular junction maintenance and gait coordination
- PMID: 39460437
- PMCID: PMC11967847
- DOI: 10.1093/brain/awae336
MBNL deficiency in motor neurons disrupts neuromuscular junction maintenance and gait coordination
Abstract
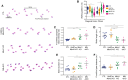
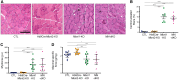
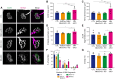
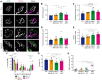
Muscleblind-like proteins (MBNLs) are a family of RNA-binding proteins that play essential roles in the regulation of RNA metabolism. Beyond their canonical role in RNA regulation, MBNL proteins have emerged as key players in the pathogenesis of myotonic dystrophy type 1. In myotonic dystrophy type 1, sequestration of MBNL proteins by expansion of the CUG repeat RNA leads to functional depletion of MBNL, resulting in deregulated alternative splicing and aberrant RNA processing, which underlie the clinical features of the disease. Although attention on MBNL proteins has focused on their functions in skeletal muscle, new evidence suggests that their importance extends to motor neurons (MNs), pivotal cellular components in the control of motor skills and movement. To address this question, we generated conditional double-knockout (dKO) mice, in which Mbnl1 and Mbnl2 were specifically deleted in motor neurons (MN-dKO). Adult MN-dKO mice develop gait coordination deficits associated with structural and ultrastructural defects in the neuromuscular junction, indicating that MBNL activity in MNs is crucial for the maintenance of the neuromuscular junction. In addition, transcriptome analysis performed on the spinal cord of MN-dKO mice identified mis-splicing events in genes associated with synaptic transmission and neuromuscular junction homeostasis. In summary, our results highlight the complex roles and regulatory mechanisms of MBNL proteins in MNs for muscle function and locomotion. This work provides valuable insights into fundamental aspects of RNA biology and offers promising avenues for therapeutic intervention in myotonic dystrophy type 1 and in a range of diseases associated with RNA dysregulation.
Keywords: MBNL; RNA binding protein; alternative splicing; motor neurons; myotonic dystrophy type 1; neuromuscular junction.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Fardaei M, Rogers MT, Thorpe HM, Larkin K, Hamshere MG, Harper PS, Brook JD Three proteins, MBNL, MBLL and MBXL, co-localize in vivo with nuclear foci of expanded-repeat transcripts in DM1 and DM2 cells. Hum Mol Genet. 2002;11:805–814. - PubMed
-
- Klein AF, Gasnier E, Furling D. Gain of RNA function in pathological cases: Focus on myotonic dystrophy. Biochimie. 2011;93:2006–2012. - PubMed
-
- Rau F, Freyermuth F, Fugier C, et al. Misregulation of miR-1 processing is associated with heart defects in myotonic dystrophy. Nat Struct Mol Biol. 2011;18:840–845. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

