Informing the risk assessment related to lactation and drug exposure: A physiologically based pharmacokinetic lactation model for pregabalin
- PMID: 39460526
- PMCID: PMC11578138
- DOI: 10.1002/psp4.13266
Informing the risk assessment related to lactation and drug exposure: A physiologically based pharmacokinetic lactation model for pregabalin
Abstract
Breastfeeding is important in childhood development, and medications are often necessary for lactating individuals, yet information on the potential risk of infant drug exposure through human milk is limited. Establishing a lactation modeling framework can advance our understanding of this topic and potentiate clinical decision making. We expanded the modeling framework previously developed for sotalol using pregabalin as a second prototypical probe compound with similar absorption, distribution, metabolism, and elimination (ADME) properties. Adult oral models were developed in PK-Sim® and used to build a lactation model in MoBi® to simulate drug transfer into human milk. The adult model was applied to breastfeeding pediatrics (ages 1 to 23 months) and subsequently integrated with the lactation model to simulate infant drug exposure according to age, size, and breastfeeding frequency. Physiologically based pharmacokinetic (PBPK) model simulations captured the data used for verification both in adults and pediatrics. Lactation simulations captured observed milk and plasma data corresponding to doses of 150 mg administered twice daily to lactating individuals, and estimated a relative infant dose (RID) of approximately 7% of the maternal dose. The infant drug exposure simulations showed peak plasma concentrations of 0.44 μg/mL occurring within the first 2 weeks of life, followed by gradual decline with age after week four. The modeling framework performs well for this second prototypical drug and warrants expansion to other drugs for further validation. PBPK modeling and simulation approaches together with clinical lactation data could ultimately help inform infant drug exposure risk assessments to guide clinical decision making.
© 2024 The Author(s). CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
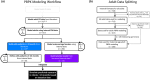
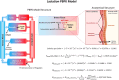

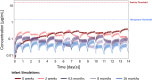
References
-
- Meek JY, Noble L. Technical report: breastfeeding and the use of human milk. Pediatrics. 2022;150(1):e2022057989. - PubMed
-
- CDC 2022 breastfeeding report card. Ctr Dis Control Prev. 2023. Accessed November 21, 2023. https://www.cdc.gov/breastfeeding/data/reportcard.htm
-
- National Center for Health Statistics . Health, United States, Table 39. 2023. Accessed November 21, 2023. https://www.cdc.gov/nchs/fastats/drug‐use‐therapeutic.htm
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

