Long-term safety and efficacy of adjunctive lacosamide in the treatment of generalized onset tonic-clonic seizures: An open-label extension trial
- PMID: 39460685
- PMCID: PMC11647428
- DOI: 10.1111/epi.18158
Long-term safety and efficacy of adjunctive lacosamide in the treatment of generalized onset tonic-clonic seizures: An open-label extension trial
Abstract
Objective: This study was undertaken to assess long-term safety, tolerability, and efficacy of lacosamide (LCM) as adjunctive therapy for generalized onset tonic-clonic seizures (GTCS) in patients aged ≥4 years with idiopathic generalized epilepsy (IGE).
Methods: EP0012 (NCT02408549) was a phase 3, multicenter, open-label extension (OLE) trial. Patients were enrolled from SP0982 (NCT02408523). Trial duration was ≥2 years (adults) and ≤5 years (children). The trial consisted of a treatment period, ≤4-week taper period, and 30-day safety follow-up. Safety (primary) variables were incidence of treatment-emergent adverse events (TEAEs), discontinuations due to TEAEs, incidence of onset of absence or myoclonic seizures, and increase in days with absence or myoclonic seizures per 28 days. Efficacy (secondary) variable was percent change in GTCS frequency per 28 days. Kaplan-Meier estimated retention rates and analyses by number of lifetime antiseizure medications (ASMs) were performed post hoc.
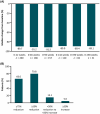
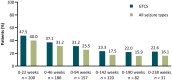
Results: Overall, 239 patients (mean age = 27.9 years, 56.1% female, 18.4% children) were enrolled and received ≥1 dose of LCM in this OLE (median treatment duration = 3.2 years); 157 (65.7%) completed the trial, and 82 (34.3%) discontinued. The most common reason for discontinuation (≥10%) was withdrawn consent (30 [12.6%]). Kaplan-Meier estimated retention rate was 87%, 72%, and 60% at 1, 3, and 5 years, respectively. Overall, 222 (92.9%) patients reported TEAEs; 19 (7.9%) discontinued due to TEAEs. Few patients had an increase in number of days with absence or myoclonic seizures, or incidence of new absence or myoclonic seizures. Median percent change in GTCS frequency per 28 days from the combined baseline was -88.6% (range = -100.0 to 465.4, n = 238). Post hoc analyses demonstrated small numerical differences between patients with 1, 2, and ≥3 lifetime ASMs.
Significance: The results support the use of long-term adjunctive LCM for GTCS in patients with IGE. Long-term adjunctive LCM was efficacious and well tolerated independent of the number of ASMs used before LCM initiation.
Clinical trial registration: ClinicalTrials.gov: NCT02408549.
Keywords: antiseizure medication; idiopathic generalized epilepsy; long‐term retention; phase 3 trial; primary generalized epilepsy; treatment‐emergent adverse events.
© 2024 UCB Biopharma SRL and The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
D.G.V. has received speaker honoraria from Eisai, Greenwich Biosciences, Lundbeck, SK Life Science, Sunovion, and UCB; his institution has received payments for his services as a principal investigator on randomized controlled trials sponsored by Biogen, Eisai, Longboard, Marinus, Pfizer, SK Life Science, UCB, and Xenon Pharmaceuticals; he has served as an advisor to Longboard, Otsuka Pharmaceutical Development and Commercialization, SK Life Science, UCB, and Xenon Pharmaceuticals. M.K.F. and I.P. report no conflicts of interest. M.W. has received speaker honoraria from Eisai, Otsuka Pharmaceutical, and UCB. P.C., S.D., C.M., R.R., and P.W. are salaried employees of UCB and have received UCB stocks from their employment. T.J.O. has received research funding from Anavex, Biogen, Eisai, Praxis Precision Medicines, UCB, and Zynerba. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures

References
-
- World Health Organization (WHO) . Epilepsy. 2024. Retrieved February 23, 2024 from: https://www.who.int/news‐room/fact‐sheets/detail/epilepsy
-
- Kanner AM, Bicchi MM. Antiseizure medications for adults with epilepsy: a review. JAMA. 2022;327:1269–1281. - PubMed
-
- World Health Organization . Epilepsy: a public health imperative. 2024. Retrieved February 23, 2024 from: https://www.who.int/publications/i/item/epilepsy‐a‐public‐health‐imperative
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

