Mouse models of Slc35a2 brain mosaicism reveal mechanisms of mild malformations of cortical development with oligodendroglial hyperplasia in epilepsy
- PMID: 39460689
- PMCID: PMC11647420
- DOI: 10.1111/epi.18166
Mouse models of Slc35a2 brain mosaicism reveal mechanisms of mild malformations of cortical development with oligodendroglial hyperplasia in epilepsy
Abstract
Objective: Brain somatic variants in SLC35A2 were recently identified as a genetic marker for mild malformations of cortical development with oligodendroglial hyperplasia in epilepsy (MOGHE). The role of SLC35A2 in cortical development and the contributions of abnormal neurons and oligodendrocytes to seizure activity in MOGHE remain largely unexplored.
Methods: Here, we generated a novel Slc35a2 floxed allele, which we used to develop two Slc35a2 conditional knockout mouse lines targeting (1) the Emx1 dorsal telencephalic lineage (excitatory neurons and glia) and (2) the Olig2 lineage (oligodendrocytes). We examined brain structure, behavior, and seizure activity.
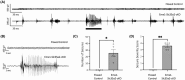
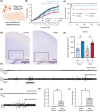
Results: Knockout of Slc35a2 from the Emx1 lineage, which targets both cortical neurons and oligodendrocytes, resulted in early lethality and caused abnormal cortical development, increased oligodendroglial cell density, early onset seizures, and developmental delays akin to what is observed in patients with MOGHE. By tracing neuronal development with 5-Ethynyl-2'-deoxyuridine (EdU) birthdating experiments, we found that Slc35a2 deficiency disrupts corticogenesis by delaying radial migration of neurons from the subventricular zone. To discern the contributions of oligodendrocytes to these phenotypes, we knocked out Slc35a2 from the Olig2 lineage. This recapitulated the increased oligodendroglial cell density and resulted in abnormal electroencephalographic activity, but without a clear seizure phenotype, suggesting Slc35a2 deficiency in neurons is required for epileptogenesis.
Significance: This study presents two novel Slc35a2 conditional knockout mouse models and characterizes the effects on brain development, behavior, and epileptogenesis. Together, these results demonstrate a direct causal role for SLC35A2 in MOGHE-like phenotypes, including a critical role in neuronal migration during brain development, and identify neurons as key contributors to SLC35A2-related epileptogenesis.
Keywords: brain mosaicism; glycosylation; malformation of cortical development; pediatrics.
© 2024 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
T.A.B. is cofounder of BehaviorCloud. The remaining authors have no conflicts of interest. The authors confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures



References
-
- Wiktor M, Wiertelak W, Maszczak‐Seneczko D, Balwierz PJ, Szulc B, Olczak M. Identification of novel potential interaction partners of UDP‐galactose (SLC35A2), UDP‐N‐acetylglucosamine (SLC35A3) and an orphan (SLC35A4) nucleotide sugar transporters. J Proteome. 2021;249:104321. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

