TGF-β antagonism synergizes with PPARγ agonism to reduce fibrosis and enhance beige adipogenesis
- PMID: 39461664
- PMCID: PMC11570741
- DOI: 10.1016/j.molmet.2024.102054
TGF-β antagonism synergizes with PPARγ agonism to reduce fibrosis and enhance beige adipogenesis
Abstract
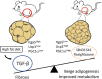
Objectives: Adipose tissue depots vary markedly in their ability to store and metabolize triglycerides, undergo beige adipogenesis and susceptibility to metabolic disease. The molecular mechanisms that underlie such heterogeneity are not entirely clear. Previously, we showed that TGF-β signaling suppresses beige adipogenesis via repressing the recruitment of dedicated beige progenitors. Here, we find that TGF-β signals dynamically regulate the balance between adipose tissue fibrosis and beige adipogenesis.
Methods: We investigated adipose tissue depot-specific differences in activation of TGF-β signaling in response to dietary challenge. RNA-seq and fluorescence activated cell sorting was performed to identify and characterize cells responding to changes in TGF-β signaling status. Mouse models, pharmacological strategies and human adipose tissue analyses were performed to further define the influence of TGF-β signaling on fibrosis and functional beige adipogenesis.
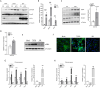
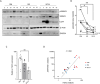
Results: Elevated basal and high-fat diet inducible activation of TGF-β/Smad3 signaling was observed in the visceral adipose tissue depot. Activation of TGF-β/Smad3 signaling was associated with increased adipose tissue fibrosis. RNA-seq combined with fluorescence-activated cell sorting of stromal vascular fraction of epididymal white adipose tissue depot resulted in identification of TGF-β/Smad3 regulated ITGA5+ fibrogenic progenitors. TGF-β/Smad3 signal inhibition, genetically or pharmacologically, reduced fibrosis and increased functional beige adipogenesis. TGF-β/Smad3 antagonized the beneficial effects of PPARγ whereas TGF-β receptor 1 inhibition synergized with actions of rosiglitazone, a PPARγ agonist, to dampen fibrosis and promote beige adipogenesis. Positive correlation between TGF-β activation and ITGA5 was observed in human adipose tissue, with visceral adipose tissue depots exhibiting higher fibrosis potential than subcutaneous or brown adipose tissue depots.
Conclusions: Basal and high-fat diet inducible activation of TGF-β underlies the heterogeneity of adipose tissue depots. TGF-β/Smad3 activation promotes adipose tissue fibrosis and suppresses beige progenitors. Together, these dual mechanisms preclude functional beige adipogenesis. Controlled inhibition of TβRI signaling and concomitant PPARγ stimulation can suppress adipose tissue fibrosis and promote beige adipogenesis to improve metabolism.
Keywords: Beige adipogenesis; Fibrosis; Metabolism; PPARγ; Rosiglitazone; Smad3; TGF-β.
Published by Elsevier GmbH.
Conflict of interest statement
Declaration of competing interest The authors have declared that no conflict of interest exists.
Figures



References
-
- Cypess A.M. Reassessing human adipose tissue. N Engl J Med. 2022;386(8):768–779. - PubMed
-
- Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. - PubMed
-
- Nedergaard J., Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metabol. 2010;11(4):268–272. - PubMed
-
- Nedergaard J., Cannon B. The browning of white adipose tissue: some burning issues. Cell Metabol. 2014;20(3):396–407. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

