Dendritic cell-intrinsic PTPN22 negatively regulates antitumor immunity and impacts anti-PD-L1 efficacy
- PMID: 39461876
- PMCID: PMC11529514
- DOI: 10.1136/jitc-2024-009588
Dendritic cell-intrinsic PTPN22 negatively regulates antitumor immunity and impacts anti-PD-L1 efficacy
Abstract
Background: Individuals with a loss-of-function single-nucleotide polymorphism in the gene encoding PTPN22 have an increased risk for autoimmune diseases, and patients with cancer with such alleles may respond better to checkpoint blockade immunotherapy. Studies in PTPN22 knockout (KO) mice have established it as a negative regulator of T cell responses in cancer models. However, the role of PTPN22 in distinct immune cell compartments, such as dendritic cells (DCs), remains undefined.
Methods: We developed a novel PTPN22 conditional KO (cKO) mouse model that enables specific deletion in CD11c+ DCs by crossing to CD11c-Cre transgenic mice. Antitumor immunity was characterized using the B16.SIY and MC38.SIY cancer models and immune profiles of relevant tissues were evaluated by spectral flow cytometry. Antigen uptake, processing, and presentation, as well as DC proliferation to Flt3L, were characterized ex vivo.
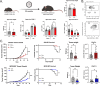
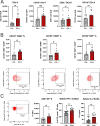
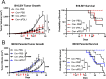
Results: Deletion of PTPN22 in DCs resulted in augmented antitumor immunity in multiple syngeneic tumor models. Tumor antigen-specific CD8+ T cells were increased in the tumor microenvironment (TME) of PTPN22 cKO mice and improved tumor control was CD8+ T cell-dependent. Augmented T cell priming was also detected at early time points in the draining lymph nodes, and these effects were correlated with an increased number of proliferating CD103+ DCs, also seen in the TME. In vitro studies revealed increased DC proliferation in response to Flt3L, as well as increased antigen processing and presentation. PTPN22 cKO mice bearing MC38 parental tumors showed combinatorial benefit with anti-PD-L1 therapy.
Conclusions: Deletion of PTPN22 in DCs is sufficient to drive an augmented tumor antigen-specific T cell response, resulting in enhanced tumor control. PTPN22 negatively regulates DC proliferation and antigen processing and presentation. Our work argues that PTPN22 is an attractive therapeutic target for cancer immunotherapy and highlights the potential to modulate antitumor immunity through the manipulation of DC signaling.
Keywords: Dendritic; Genetic; Immunotherapy; Myeloid; Solid tumor.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
