Safety and efficacy of Mg-Dy membrane with poly-L-lactic acid coating for guided bone regeneration
- PMID: 39462023
- PMCID: PMC11513034
- DOI: 10.1038/s41598-024-77211-1
Safety and efficacy of Mg-Dy membrane with poly-L-lactic acid coating for guided bone regeneration
Abstract
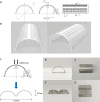
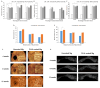
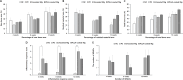
The aim of this study was to evaluate safety and efficacy of a poly-L-lactic acid (PLLA)-coated magnesium (Mg)-Dysprosium (Dy) membrane in guided bone regeneration (GBR) using a rabbit calvarium model. The microstructure of the Mg-Dy membrane surface and thickness of the PLLA coating were examined. In vitro degradation and cytotoxicity test was conducted. The in vivo study used 24 white male rabbits with two 8 mm-diameter defects created on the calvaria; 12 defects were randomly assigned per group: (1) Negative control, (2) positive control, (3) uncoated Mg, and (4) PLLA-coated Mg group. Specimens were harvested at 4, 8, and 12 weeks postoperatively for radiological, histological, and histomorphometric analyses. The PLLA-coated Mg-Dy membrane showed a low degree of degradation, indicating that the coating exerted a protective effect. In the cytotoxicity test, no deformed or degenerated cells were observed. In the in vivo study, radiographic and histomorphometric analyses indicated favorable new bone formation and maintenance of the graft material for PLLA-coated Mg group. PLLA-coated Mg group, compared to the uncoated counterpart, restored the bony contour more completely, without inducing significant inflammatory response. Our results support the safety and efficacy of PLLA-coated Mg-Dy membranes for GBR both in vitro and in vivo.
Keywords: Biocompatible coated material; Biodegradable membrane; Dental implant; Guided tissue regeneration; Magnesium; New Zealand white rabbit.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Poly (l-lactic acid) membrane crosslinked with Genipin for guided bone regeneration.Int J Biol Macromol. 2021 Nov 30;191:1228-1239. doi: 10.1016/j.ijbiomac.2021.09.137. Epub 2021 Oct 4. Int J Biol Macromol. 2021. PMID: 34619279
-
Magnesium Resorbable Membrane for Guided Bone Regeneration in Critical Size Defect Model in Rabbits-Histomorphometric Analysis.Clin Implant Dent Relat Res. 2025 Jun;27(3):e70055. doi: 10.1111/cid.70055. Clin Implant Dent Relat Res. 2025. PMID: 40471118 Free PMC article.
-
Biodegradable Magnesium alloy Janus membrane with surface-selective osteoinduction and soft tissue healing properties in guided bone regeneration.Acta Biomater. 2025 Mar 15;195:582-598. doi: 10.1016/j.actbio.2025.02.001. Epub 2025 Feb 9. Acta Biomater. 2025. PMID: 39933642
-
Preparation of thermoplastic poly(L-lactic acid) membranes for guided bone regeneration.Int J Oral Maxillofac Implants. 2013 Jul-Aug;28(4):973-81. doi: 10.11607/jomi.2729. Int J Oral Maxillofac Implants. 2013. PMID: 23869354
-
Research status and prospects of biodegradable magnesium-based metal guided bone regeneration membranes.Hua Xi Kou Qiang Yi Xue Za Zhi. 2024 Aug 1;42(4):415-425. doi: 10.7518/hxkq.2024.2024140. Hua Xi Kou Qiang Yi Xue Za Zhi. 2024. PMID: 39049628 Free PMC article. Review. Chinese, English.
Cited by
-
The role of pH on structure, corrosion behavior and biocompatibility of MgFe layered double hydroxide coating on Mg-Nd-Zn-Zr alloy.Sci Rep. 2025 Apr 28;15(1):14842. doi: 10.1038/s41598-025-98555-2. Sci Rep. 2025. PMID: 40295569 Free PMC article.
References
-
- Cho, K. S. et al. Alveolar bone formation at dental implant dehiscence defects following guided bone regeneration and xenogeneic freeze-dried demineralized bone matrix. Clin. Oral. Implants. Res. 9, 419–428. 10.1034/j.1600-0501.1996.090607.x (1998). - PubMed
-
- Simion, M. in Proceedings of the 3rd European Workshop on Periodontology. 500–519 (Quintessence Publishing Co).
-
- Klokkevold, P. R. & Newman, M. G. Current status of dental implants: a periodontal perspective. Int. J. Oral Maxillofac. Implants. 15, 56–65 (2000). - PubMed
-
- Walker, J., Shadanbaz, S., Woodfield, T. B., Staiger, M. P. & Dias, G. J. Magnesium biomaterials for orthopedic application: a review from a biological perspective. J. Biomed. Mater. Res. B. 102, 1316–1331. 10.1002/jbm.b.33113 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

