Histamine stimulates human microglia to alter cellular prion protein expression via the HRH2 histamine receptor
- PMID: 39462031
- PMCID: PMC11513956
- DOI: 10.1038/s41598-024-75982-1
Histamine stimulates human microglia to alter cellular prion protein expression via the HRH2 histamine receptor
Abstract
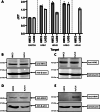
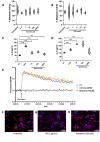
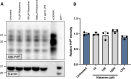
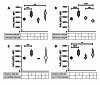
Although the cellular prion protein (PrPC) has been evolutionarily conserved, the role of this protein remains elusive. Recent evidence indicates that PrPC may be involved in neuroinflammation and the immune response in the brain, and its expression may be modified via various mechanisms. Histamine is a proinflammatory mediator and neurotransmitter that stimulates numerous cells via interactions with histamine receptors 1-4 (HRH1-4). Since microglia are the innate immune cells of the central nervous system, we hypothesized that histamine-induced stimulation regulates the expression of PrPC in human-derived microglia. The human microglial clone 3 (HMC3) cell line was treated with histamine, and intracellular calcium levels were measured via a calcium flux assay. Cytokine production was monitored by enzyme-linked immunosorbent assay (ELISA). Western blotting and quantitative reverse transcription-polymerase chain reaction were used to determine protein and gene expression of HRH1-4. Flow cytometry and western blotting were used to measure PrPC expression levels. Fluorescence microscopy was used to examine Iba-1 and PrPC localization. HMC3 cells stimulated by histamine exhibited increased intracellular calcium levels and increased release of IL-6 and IL-8, while also modifying PrPC localization. HMC3 stimulated with histamine for 6 and 24 hours exhibited increased surface PrPC expression. Specifically, we found that stimulation of the HRH2 receptor was responsible for changes in surface PrPC. Histamine-induced increases in surface PrPC were attenuated following inhibition of the HRH2 receptor via the HRH2 antagonist ranitidine. These changes were unique to HRH2 activation, as stimulation of HRH1, HRH3, or HRH4 did not alter surface PrPC. Prolonged stimulation of HMC3 decreased PrPC expression following 48 and 72 hours of histamine stimulation. HMC3 cells can be stimulated by histamine to undergo intracellular calcium influx. Surface expression levels of PrPC on HMC3 cells are altered by histamine exposure, primarily mediated by HRH2. While histamine exposure also increases release of IL-6 and IL-8 in these cells, this cytokine release is not fully dependent on PrPC levels, as IL-6 release is only partially reduced and IL-8 release is unchanged under the conditions of HRH2 blockade that prevent PrPC changes. Overall, this suggests that PrPC may play a role in modulating microglial responses.
Keywords: Degranulation; Histamine; Mast cell; Microglia; Neuroinflammation; Prion protein.
© 2024. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

