Reentrant DNA shells tune polyphosphate condensate size
- PMID: 39462120
- PMCID: PMC11513989
- DOI: 10.1038/s41467-024-53469-x
Reentrant DNA shells tune polyphosphate condensate size
Abstract
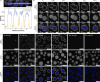
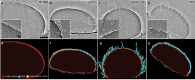
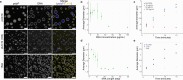
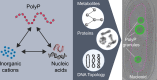
The inorganic biopolymer polyphosphate (polyP) occurs in all domains of life and affects myriad cellular processes. A longstanding observation is polyP's frequent proximity to chromatin, and, in many bacteria, its occurrence as magnesium (Mg2+)-enriched condensates embedded in the nucleoid region, particularly in response to stress. The physical basis of the interaction between polyP, DNA and Mg2+, and the resulting effects on the organization of the nucleoid and polyP condensates, remain poorly understood. Here, using a minimal system of polyP, Mg2+, and DNA, we find that DNA can form shells around polyP-Mg2+ condensates. These shells show reentrant behavior, that is, they form within a window of Mg2+ concentrations, representing a tunable architecture with potential relevance in other multicomponent condensates. This surface association tunes condensate size and DNA morphology in a manner dependent on DNA length and concentration, even at DNA concentrations orders of magnitude lower than found in the cell. Our work also highlights the remarkable capacity of two primordial inorganic species to organize DNA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Update of
-
Reentrant DNA shells tune polyphosphate condensate size.bioRxiv [Preprint]. 2023 Sep 15:2023.09.13.557044. doi: 10.1101/2023.09.13.557044. bioRxiv. 2023. Update in: Nat Commun. 2024 Oct 26;15(1):9258. doi: 10.1038/s41467-024-53469-x. PMID: 37745474 Free PMC article. Updated. Preprint.
References
-
- Rao, N. N., Gómez-García, M. R. & Kornberg, A. Inorganic polyphosphate: essential for growth and survival. Annu. Rev. Biochem.78, 605–647 (2009). - PubMed
-
- Denoncourt, A. & Downey, M. Model systems for studying polyphosphate biology: a focus on microorganisms. Curr. Genet.67, 331–346 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- S10 OD032467/OD/NIH HHS/United States
- DP2-GM-739-140918/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- Award #903967/American Heart Association (American Heart Association, Inc.)
- Pew Scholars Program/Pew Charitable Trusts
- R35 GM130375/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

