Tasurgratinib in patients with cholangiocarcinoma or gastric cancer: Expansion part of the first-in-human phase I study
- PMID: 39462221
- PMCID: PMC11711049
- DOI: 10.1111/cas.16354
Tasurgratinib in patients with cholangiocarcinoma or gastric cancer: Expansion part of the first-in-human phase I study
Abstract
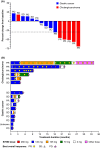
Fibroblast growth factor receptors (FGFRs) are a highly conserved family of transmembrane receptor tyrosine kinases with multiple roles in the regulation of key cellular processes. Specific FGFR mutations have been observed in several types of cancers, including gastric carcinoma and cholangiocarcinoma. Dose escalation data of 24 Japanese patients with solid tumors treated with Tasurgratinib (previously known as E7090), a potent, selective FGFR1-3 inhibitor, was reported in a phase I, first-in-human, single-center study. Based on the safety, pharmacokinetic, and pharmacodynamic profiles observed in this study, the recommended dose of 140 mg once daily was selected for the expansion part (Part 2), a multicenter expansion of the dose-finding study restricted to patients with tumors harboring FGFR gene alterations. Safety and preliminary efficacy were assessed in Part 2. Pharmacodynamic pharmacogenomic markers (serum phosphate, FGF23, and 1,25-(OH)2-vitamin D, circulating tumor DNA) and pharmacokinetic profiles were also evaluated. A total of 16 patients were enrolled in Part 2, six with cholangiocarcinoma and 10 with gastric cancer. The most common treatment-emergent adverse events were hyperphosphatemia, palmar-plantar erythrodysesthesia syndrome, and paronychia. Five partial responses (83.3%) in cholangiocarcinoma patients and one partial response (11.1%) in gastric cancer patients were observed; median progression-free survival was 8.26 months (95% confidence interval [CI] 3.84, not evaluable [NE]) and 3.25 months (95% CI 0.95, 4.86), and overall survival was 22.49 months (95% CI 6.37, NE) and 4.27 months (95% CI 2.23, 7.95), respectively, in the two groups. In conclusion, Tasurgratinib 140 mg has a tolerable safety profile with good clinical efficacy in patients with cholangiocarcinoma harboring FGFR2 gene rearrangements.
Keywords: E7090; FGFR2; Tasurgratinib; cholangiocarcinoma; gastric cancer.
© 2024 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
C. Morizane received personal fees from Astra Zeneca, Taiho Pharmaceutical, and Nihon Servier and research funds from Eisai, Yakult, Ono Pharmaceutical, Taiho Pharmaceutical, J‐Pharma, AstraZeneca, Merck Biopharma, Daiichi Sankyo, and Boehringer Ingelheim. M. Ueno received personal fees from Taiho Pharmaceutical, AstraZeneca, K.K, Yakult Honsha, Nihon Servier, Incyte Biosciences Japan GK, and Chugai Pharmaceutical, and research funds from Taiho Pharmaceutical, AstraZeneca, MSD K.K, Nihon Servier, Ono Pharmaceutical, Incyte Biosciences Japan Company, Chugai Pharmaceutical, Boehringer Ingelheim GmbH, J‐Pharma, Eisai, Novartis Pharma K.K, Astellas Pharma Inc., DFP (Delta Fly Pharma), Novocure GmbH, and Chiome Bioscience. T. Ioka received personal fees from Taiho and Astra Zeneca. M. Ikeda received fees from AstraZeneca, Chugai Pharma, Eisai, Incyte, Lilly Japan, MSD, Novartis, Ono Pharmaceutical, Takeda, Teijin Pharma, Nihon Servier, Taiho Pharmaceutical; research funds from Astra Zeneca, Bayer, Bristol‐Myers Squibb, Chiome Bioscience, Chugai, Eisai, Eli Lilly Japan, Delta‐Fly Pharma, Invitae, J‐Pharma, Merck Biopharma, Merus N.V., MSD, Novartis, Nihon Servier, Ono Pharmaceutical, Syneos Health, and Rakuten Medical, and is an editorial board member of
Figures


References
-
- Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017;17:318‐332. - PubMed
-
- Wesche J, Haglund K, Haugsten EM. Fibroblast growth factors and their receptors in cancer. Biochem J. 2011;437:199‐213. - PubMed
-
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116‐129. - PubMed
-
- Courjal F, Cuny M, Simony‐Lafontaine J, et al. Mapping of DNA amplifications at 15 chromosomal localizations in 1875 breast tumors: definition of phenotypic groups. Cancer Res. 1997;57:4360‐4367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

