Microaxial Flow Pump Hemodynamic and Metabolic Effects in Infarct-Related Cardiogenic Shock: A Substudy of the DanGer Shock Randomized Clinical Trial
- PMID: 39462240
- PMCID: PMC11513791
- DOI: 10.1001/jamacardio.2024.4197
Microaxial Flow Pump Hemodynamic and Metabolic Effects in Infarct-Related Cardiogenic Shock: A Substudy of the DanGer Shock Randomized Clinical Trial
Abstract
Importance: Mechanical circulatory support with a microaxial flow pump (MAFP) has been shown to improve survival in ST-elevation myocardial infarction-induced cardiogenic shock (STEMI-CS). Understanding the impact on hemodynamic stability over time is crucial for optimizing patient treatment.
Objective: To determine if an MAFP reduces the need for pharmacological circulatory support without compromising hemodynamics compared with standard care in STEMI-CS.
Design, setting, and participants: This was a substudy of the Danish-German (DanGer) Shock trial, an international, multicenter, open-label randomized clinical trial. Patients from 14 heart centers across Denmark, Germany, and the UK were enrolled. Inclusion criteria for the trial were STEMI and systolic blood pressure less than 100 mm Hg or ongoing vasopressor treatment, left ventricular ejection fraction less than 45%, and arterial lactate level greater than 2.5 mmol/L. Of the enrolled patients, after exclusions from death in the catheterization laboratory or immediately on intensive care unit (ICU) admission, the remaining patients had serial recordings of hemodynamics, arterial lactate, and use of vasoactive drugs. Patients who were in comas after cardiac arrest and patients with mechanical complications or right ventricular failure were excluded. Data were analyzed from May to September 2024.
Interventions: MAFP and standard of care or standard of care alone.
Main outcomes and measures: Hemodynamic status in terms of heart rate and blood pressure, metabolic status in terms of arterial lactate concentration, and vasoactive-inotropic score (VIS). The clinical events during the first 72 hours were as follows: death from all causes, escalation of mechanical circulatory support, and discharge alive from the ICU.
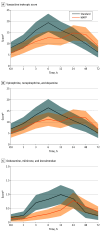
Results: From 355 enrolled patients, 324 (mean [IQR] age, 68 [58-75] years; 259 male [80%]) underwent ICU treatment (169 [52%] in the MAFP group, 155 [48%] in the standard-care group). Baseline characteristics were balanced. There was no difference in heart rate between groups, and mean arterial pressure was above the treatment target of 65 mm Hg in both groups but was achieved with a lower VIS in the MAFP group. No difference in arterial lactate level was found between groups at randomization, but on arrival to the ICU, the MAFP group had significantly lower arterial lactate levels compared with the standard-care group (mean difference, 1.3 mmol/L; 95% CI, 0.7-1.9 mmol/L), a difference that persisted throughout the first 24 hours of observation. The MAFP group achieved lactate normalization (<2 mmol/L) 12 hours (95% CI, 5-18 hours) before the standard-care group.
Conclusions and relevance: Use of a MAFP reduces the use of vasopressors and inotropic medication while maintaining hemodynamic stability and achieving faster normalization of lactate level in patients with STEMI-CS.
Trial registration: ClinicalTrials.gov Identifier: NCT01633502.
Conflict of interest statement
Figures


Comment in
-
Evidence for Mechanical Circulatory Support in Cardiogenic Shock.JAMA Cardiol. 2025 Jan 1;10(1):7-8. doi: 10.1001/jamacardio.2024.4225. JAMA Cardiol. 2025. PMID: 39462239 No abstract available.
References
-
- Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock—SHOCK Investigators: should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341(9):625-634. doi: 10.1056/NEJM199908263410901 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

