Fractional Flow Reserve and Instantaneous Wave-Free Ratio as Predictors of the Placebo-Controlled Response to Percutaneous Coronary Intervention in Stable Coronary Artery Disease
- PMID: 39462291
- PMCID: PMC11748910
- DOI: 10.1161/CIRCULATIONAHA.124.072281
Fractional Flow Reserve and Instantaneous Wave-Free Ratio as Predictors of the Placebo-Controlled Response to Percutaneous Coronary Intervention in Stable Coronary Artery Disease
Abstract
Background: ORBITA-2 (the Placebo-Controlled Trial of Percutaneous Coronary Intervention for the Relief of Stable Angina) provided evidence for the role of percutaneous coronary intervention (PCI) for angina relief in stable coronary artery disease. Fractional flow reserve (FFR) and instantaneous wave-free ratio (iFR) are often used to guide PCI; however, their ability to predict placebo-controlled angina improvement is unknown.
Methods: Participants with angina, ischemia, and stable coronary artery disease were enrolled, and anti-anginal medications were stopped. Participants reported angina episodes daily for 2 weeks using the ORBITA smartphone symptom application (ORBITA-app). At the research angiogram, FFR and iFR were measured. After sedation and auditory isolation, participants were randomized to PCI or placebo before entering a 12-week blinded follow-up phase with daily angina reporting. The ability of FFR and iFR, analyzed as continuous variables, to predict the placebo-controlled effect of PCI was tested using Bayesian proportional odds modeling.
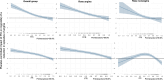
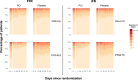
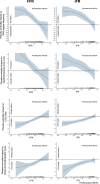
Results: Invasive physiology data were available for 279 patients (140 PCI and 139 placebo). The median (interquartile range) age was 65 years (59.0-70.5), and 223 (79.9%) were male. Median FFR was 0.60 (0.46-0.73), and median iFR was 0.76 (0.50-0.86). The lower the FFR or iFR, the greater the placebo-controlled improvement with PCI across all end points. There was strong evidence that a patient with an FFR at the lower quartile would have a greater placebo-controlled improvement in angina symptom score with PCI than a patient at the upper quartile (FFR, 0.46 versus 0.73: odds ratio, 2.01; 95% credible interval, 1.79-2.26; probability of interaction, >99.9%). Similarly, there was strong evidence that a patient with an iFR at the lower quartile would have greater placebo-controlled improvement in angina symptom score with PCI than a patient with an iFR at the upper quartile (iFR, 0.50 versus 0.86: odds ratio, 2.13; 95% credible interval, 1.87-2.45; probability of interaction, >99.9%). The relationship between benefit and physiology was seen in both Rose angina and Rose nonangina.
Conclusions: Physiological stenosis severity, as measured by FFR and iFR, predicts placebo-controlled angina relief from PCI. Invasive coronary physiology can be used to target PCI to those patients who are most likely to experience benefit.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03742050.
Keywords: angina, stable; cardiovascular physiology; clinical trials, randomized; coronary artery disease; ischemia, myocardial.
Conflict of interest statement
Dr Foley has received speaker fees from Menarini and has received consulting and speaker fees from Shockwave Medical, Inc, and Philips. Dr Rajkumar has received speaker fees from Menarini, has received consulting fees from Philips, and has received shares in Mycardium AI. Dr Simader has received a sponsorship from Servier Pharmaceuticals. Dr Khokhar has received speaker fees and travel support from Boston Scientific and Abbott. Dr Davies has received grants from Medtronic and Abbott; has received sponsorship from Vascular Perspectives, Boston Scientific, Medtronic, and Abbott; and has received speaker fees from AstraZeneca, Pfizer, Bristol Myers Squibb, and Novartis. Dr Keeble has served on advisory boards for Abbott Vascular and SMT; and has received institutional research funding from Terumo, Medtronic, Boston Scientific, Abbott Vascular, Philips Volcano, and Cardionovum. Dr O’Kane has received speaker fees from Abbott Vascular, Biosensors, Boston Scientific, Heartflow, Medtronic, Philips, Shockwave, and Terumo. Dr Kotecha has received honoraria from Bayer and Janssen. Dr Nijjer has received speaker fees from Philips Volcano, Pfizer, Bayer, AstraZeneca, Boehringer Ingelheim, and Amarin. Dr Spratt has received speaker fees from Boston Scientific Corporation and Shockwave Medical, Inc. Dr Sen has received speaker and consulting fees from Philips, Medtronic, Recor, and AstraZeneca. Dr Curzen has received grants from Beckman Coulter, Inc, Boston Scientific Corporation, Haemonetics Corporation, and HeartFlow Inc; and has received speaker fees from Heartflow. Dr Howard has received shares in Mycardium AI; and has received a grant from the British Heart Foundation. Dr Cole has received shares in Mycardium AI. Dr Al-Lamee has served on advisory boards for Janssen Pharmaceuticals, Abbott, and Philips; and has received speaker fees from Abbott, Philips, Medtronic, Servier, Omniprex, and Menarini. The other authors report no conflicts.
Figures

Comment in
-
In Which Patients Will Percutaneous Coronary Intervention Relieve Angina?Circulation. 2025 Jan 21;151(3):215-217. doi: 10.1161/CIRCULATIONAHA.124.072466. Epub 2024 Oct 27. Circulation. 2025. PMID: 39462289 No abstract available.
References
-
- Al-Lamee R, Thompson D, Dehbi HM, Sen S, Tang K, Davies J, Keeble T, Mielewczik M, Kaprielian R, Malik IS, et al. ; ORBITA Investigators. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomized controlled trial. Lancet. 2018;391:31–40. doi: 10.1016/S0140-6736(17)32714-9 - PubMed
-
- National Institute for Health and Care Excellence (NICE). Management of stable angina. CG126. 2016. Accessed October 23, 2024. https://www.nice.org.uk/guidance/cg126 - PubMed
-
- Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, Dixon DL, Fearon WF, Hess B, Johnson HM, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023;148:e9–e119. doi: 10.1161/CIR.0000000000001168 - PubMed
-
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. ; ESC Scientific Document Group. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425 - PubMed
-
- Al-Lamee R, Howard JP, Shun-Shin MJ, Thompson D, Dehbi HM, Sen S, Nijjer S, Petraco R, Davies J, Keeble T, et al. Fractional flow reserve and instantaneous wave-free ratio as predictors of the placebo-controlled response to percutaneous coronary intervention in stable single-vessel coronary artery disease: physiology-stratified analysis of ORBITA. Circulation. 2018;138:1780–1792. doi: 10.1161/CIRCULATIONAHA.118.033801 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

