Long-Term Results from an Open-Label Extension Study of Atacicept for the Treatment of IgA Nephropathy
- PMID: 39462308
- PMCID: PMC7616790
- DOI: 10.1681/ASN.0000000541
Long-Term Results from an Open-Label Extension Study of Atacicept for the Treatment of IgA Nephropathy
Abstract
Background: B-cell activating factor (BAFF) and A proliferation-inducing ligand (APRIL) play key roles in the pathogenesis of IgA nephropathy. Atacicept is a novel fully humanized fusion protein, self-administered at home by subcutaneous injection, that binds and inhibits BAFF and APRIL. By inhibiting BAFF and APRIL, atacicept targets the underlying B-cell–mediated pathogenesis driving disease progression. This study evaluated the long-term efficacy and safety of atacicept in patients with IgA nephropathy over 96 weeks.
Methods: Participants with IgA nephropathy who received atacicept (25, 75, or 150 mg) or placebo in a 36-week phase 2b, randomized, blinded trial were enrolled in an open-label extension study and received atacicept 150 mg for an additional 60 weeks. Key efficacy outcomes were changes in galactose-deficient IgA1 (Gd-IgA1), percentage of participants with hematuria, urine protein-creatinine ratio (UPCR), and eGFR over 96 weeks. Long-term safety data were also evaluated.
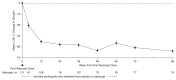
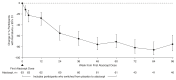
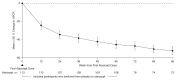
Results: There were 113 participants (67 [59%] male; 46 [41%] female) who ranged in age from 18 to 67 years who received ≥1 atacicept dose. Over 96 weeks, safety data demonstrated that atacicept was generally well tolerated. There were also sustained reductions (mean±SEM) in Gd-IgA1 (−66%±2%), percentage of participants with hematuria (−75%; 95% confidence intervals, −87 to −59; in participants with baseline hematuria), and UPCR (−52%±5%). The mean annualized slope of eGFR was −0.6±0.5 ml/min per 1.73 m2 through 96 weeks.
Conclusions: Atacicept was also well tolerated over the duration of the study. Atacicept treatment reduced Gd-IgA1, hematuria, and UPCR with stabilization of eGFR through 96 weeks.
Clinical Trial registry name and registration number:
Atacicept in Subjects with IgA Nephropathy (ORIGIN 3),
Figures

References
-
- Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol. 2005;16(7):2088–2097. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

