Glaser-Hay-Coupled Random Copolymers Containing Boron Difluoride Formazanate Dyes
- PMID: 39462480
- PMCID: PMC11841661
- DOI: 10.1002/marc.202400786
Glaser-Hay-Coupled Random Copolymers Containing Boron Difluoride Formazanate Dyes
Abstract
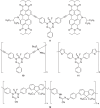
𝜋-Conjugated polymers, including those based on acetylenic repeating units, are an exciting class of materials that offer narrow optical band gaps and tunable frontier orbital energies that lead to their use in organic electronics. This work expands the knowledge of structure-property relationships of acetylenic polymers through the synthesis and characterization of a series of Glaser-Hay-coupled model compounds and random copolymers comprised of BF2 formazanate, fluorene, and/or bis(alkoxy)benzene units. The model compounds and copolymers synthesized exhibit redox activity associated with the reversible reduction of the BF2 formazanate units and the irreversible reduction of the fluorene and bis(alkoxy)benzene units. The copolymers exhibit absorption profiles characteristic or intermediate of their respective models and homopolymers, leading to broad absorption of UV-vis light. The alkyne linkages of the model compounds and copolymers are reacted with [Co2(CO)8] to convert the alkyne functional groups into cobalt carbonyl clusters. This transformation leads to blue-shifted absorption profiles due to a decrease in π-conjugation, demonstrating the ability to tune the properties of these materials through post-polymerization functionalization. The redox activity and broad absorption bands of the polymers reported make them excellent candidates for use in photovoltaics and other light-harvesting applications.
Keywords: BF2 formazanate dyes; Glaser‐Hay Coupling; UV–vis spectroscopy; alkynes; cyclic voltammetry.
© 2024 The Author(s). Macromolecular Rapid Communications published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Heeger A. J., J. Phys. Chem. B. 2001, 105, 8475.
-
- Dou L., Liu Y., Hong Z., Li G., Yang Y., Chem. Rev. 2015, 115, 12633. - PubMed
-
- Zhao X., Zhan X., Chem. Soc. Rev. 2011, 40, 3728. - PubMed
-
- Koezuka H., Tsumura A., Ando T., Synth. Met. 1987, 18, 699.
-
- Yu G., Gao J., Hummelen J. C., Wudl F., Heeger A. J., Science 1995, 270, 1789.
MeSH terms
Substances
Grants and funding
- CGS-M Scholarship/Natural Sciences and Engineering Council (NSERC) of Canada
- CGS-D Scholarship/Natural Sciences and Engineering Council (NSERC) of Canada
- DG RGPIN-2023-03318/Natural Sciences and Engineering Council (NSERC) of Canada
- Ontario Graduate Scholarship/Ontario Ministry for Research and Innovation
- JELF 33977/Canadian Foundation for Innovation
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

