Transition from fetal to postnatal state in the heart: Crosstalk between metabolism and regeneration
- PMID: 39463005
- PMCID: PMC11659109
- DOI: 10.1111/dgd.12947
Transition from fetal to postnatal state in the heart: Crosstalk between metabolism and regeneration
Abstract
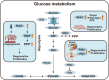
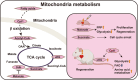
Cardiovascular disease is the leading cause of mortality worldwide. Myocardial injury resulting from ischemia can be fatal because of the limited regenerative capacity of adult myocardium. Mammalian cardiomyocytes rapidly lose their proliferative capacities, with only a small fraction of adult myocardium remaining proliferative, which is insufficient to support post-injury recovery. Recent investigations have revealed that this decline in myocardial proliferative capacity is closely linked to perinatal metabolic shifts. Predominantly glycolytic fetal myocardial metabolism transitions towards mitochondrial fatty acid oxidation postnatally, which not only enables efficient production of ATP but also causes a dramatic reduction in cardiomyocyte proliferative capacity. Extensive research has elucidated the mechanisms behind this metabolic shift, as well as methods to modulate these metabolic pathways. Some of these methods have been successfully applied to enhance metabolic reprogramming and myocardial regeneration. This review discusses recently acquired insights into the interplay between metabolism and myocardial proliferation, emphasizing postnatal metabolic transitions.
Keywords: BCAA; amino acids; cardiac metabolism; environment shift; fatty acid oxidation; glycolysis; heart regeneration; ketone body; mTOR; mitochondria.
© 2024 The Author(s). Development, Growth & Differentiation published by John Wiley & Sons Australia, Ltd on behalf of Japanese Society of Developmental Biologists.
Figures

Similar articles
-
Malonate Promotes Adult Cardiomyocyte Proliferation and Heart Regeneration.Circulation. 2021 May 18;143(20):1973-1986. doi: 10.1161/CIRCULATIONAHA.120.049952. Epub 2021 Mar 5. Circulation. 2021. PMID: 33666092 Free PMC article.
-
RNA-Binding Protein LIN28a Regulates New Myocyte Formation in the Heart Through Long Noncoding RNA-H19.Circulation. 2023 Jan 24;147(4):324-337. doi: 10.1161/CIRCULATIONAHA.122.059346. Epub 2022 Oct 31. Circulation. 2023. PMID: 36314132 Free PMC article.
-
Endocrine Influence on Cardiac Metabolism in Development and Regeneration.Endocrinology. 2021 Sep 1;162(9):bqab081. doi: 10.1210/endocr/bqab081. Endocrinology. 2021. PMID: 33880553 Free PMC article. Review.
-
Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation.J Cardiovasc Pharmacol. 2010 Aug;56(2):130-40. doi: 10.1097/FJC.0b013e3181e74a14. J Cardiovasc Pharmacol. 2010. PMID: 20505524 Review.
-
mTORC1 regulates the metabolic switch of postnatal cardiomyocytes during regeneration.J Mol Cell Cardiol. 2024 Feb;187:15-25. doi: 10.1016/j.yjmcc.2023.12.004. Epub 2023 Dec 23. J Mol Cell Cardiol. 2024. PMID: 38141532 Free PMC article.
Cited by
-
Transcriptome-Wide Insights: Neonatal Lactose Intolerance Promotes Telomere Damage, Senescence, and Cardiomyopathy in Adult Rat Heart.Int J Mol Sci. 2025 Feb 13;26(4):1584. doi: 10.3390/ijms26041584. Int J Mol Sci. 2025. PMID: 40004050 Free PMC article.
References
-
- Abouleisa, R. R. E. , McNally, L. , Salama, A. B. M. , Hammad, S. K. , Ou, Q. , Wells, C. , Lorkiewicz, P. K. , Bolli, R. , Mohamed, T. M. A. , & Hill, B. G. (2021). Cell cycle induction in human cardiomyocytes is dependent on biosynthetic pathway activation. Redox Biology, 46, 102094. 10.1016/j.redox.2021.102094 - DOI - PMC - PubMed
-
- Ali, S. R. , Nguyen, N. U. N. , Menendez‐Montes, I. , Hsu, C.‐C. , Elhelaly, W. , Lam, N. T. , Li, S. , Elnwasany, A. , Nakada, Y. , Thet, S. , Foo, R. S. Y. , & Sadek, H. A. (2024). Hypoxia‐induced stabilization of HIF2A promotes cardiomyocyte proliferation by attenuating DNA damage. The Journal of Cardiovascular Aging, 4(1), 11. 10.20517/jca.2023.43 - DOI - PMC - PubMed
-
- Arima, Y. , Nakagawa, Y. , Takeo, T. , Ishida, T. , Yamada, T. , Hino, S. , Nakao, M. , Hanada, S. , Umemoto, T. , Suda, T. , Sakuma, T. , Yamamoto, T. , Watanabe, T. , Nagaoka, K. , Tanaka, Y. , Kawamura, Y. K. , Tonami, K. , Kurihara, H. , Sato, Y. , … Tsujita, K. (2021). Murine neonatal ketogenesis preserves mitochondrial energetics by preventing protein hyperacetylation. Nature Metabolism, 3(2), 196–210. 10.1038/s42255-021-00342-6 - DOI - PubMed
-
- Aubert, G. , Martin, O. J. , Horton, J. L. , Lai, L. , Vega, R. B. , Leone, T. C. , Koves, T. , Gardell, S. J. , Krüger, M. , Hoppel, C. L. , Lewandowski, E. D. , Crawford, P. A. , Muoio, D. M. , & Kelly, D. P. (2016). The failing heart relies on ketone bodies as a fuel. Circulation, 133(8), 698–705. 10.1161/CIRCULATIONAHA.115.017355 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- Bristol-Myers Squibb
- Naito Foundation
- 17H05083/Japan Society for the Promotion of Science
- 19K22629/Japan Society for the Promotion of Science
- 20H03680/Japan Society for the Promotion of Science
- 21K18273/Japan Society for the Promotion of Science
- Japan Agency for Medical Research and Development, PRIME
- Mochida Memorial Foundation for Medical and Pharmaceutical Research
- Toray Science Foundation
- Princess Takamatsu Cancer Research Fund
- Mitsubishi Foundation
- Japanese Circulation Society
- Senri Life Science Foundation
- Hoansha Foundation
- JPMJSP2138/JST SPRING
- Takeda Science Foundation
- Uehara Memorial Foundation
- JPMJFR210O/JST FOREST
LinkOut - more resources
Full Text Sources
Miscellaneous

