The arginine/ornithine binding protein ArgT plays an essential role in Brucella neotomae/ Brucella melitensis to prevent intracellular killing and contribute to chronic persistence in the host
- PMID: 39463062
- PMCID: PMC11540086
- DOI: 10.1080/21505594.2024.2421983
The arginine/ornithine binding protein ArgT plays an essential role in Brucella neotomae/ Brucella melitensis to prevent intracellular killing and contribute to chronic persistence in the host
Abstract
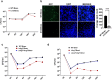
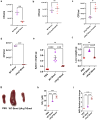
Brucella species are facultative intracellular bacterial pathogens that cause the contagious zoonotic disease, brucellosis. Brucella spp. infect a wide range of animals, including livestock, wild animals, and marine mammals. Compared with other invasive bacterial pathogens, partial information is available on the virulence factors of Brucella that enable them to survive in the host. Here, we performed transposon-based random mutagenesis of B. neotomae and identified the arginine/ornithine binding protein, ArgT, as one of the crucial virulence determinants of Brucella. Deleting ArgT from B. neotomae or B. melitensis resulted in its attenuation in macrophages, which was restored upon complementation with an ArgT expression plasmid. We observed that macrophages infected with ΔArgT-B. neotomae produced elevated levels of NO due to the inability of these mutants to deplete the host intracellular arginine through their importer. Furthermore, defective survival of ΔArgT B. neotomae and B. melitensis was observed in the infected mice, which correlated with enhanced NO production in the mice. Our studies revealed that ArgT plays a vital role in preventing intracellular killing and contributes to the chronic persistence of B. neotomae/B. melitensis in the host. This study highlights the essential role of arginine in clearing intracellular infections and the subversion of this host defense mechanism by intracellular pathogens for their chronic persistence.
Keywords: ArgT; Brucella; arginine; attenuation; iNOS; nitric oxide.
Figures


References
-
- Nicoletti P. Brucellosis: past, present and future. Prilozi. 2010;31(1):21–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
