Immunotherapy Vaccines for Prostate Cancer Treatment
- PMID: 39463159
- PMCID: PMC11513549
- DOI: 10.1002/cam4.70294
Immunotherapy Vaccines for Prostate Cancer Treatment
Abstract
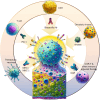
Background: Therapeutic tumor vaccines have emerged as a compelling avenue for treating patients afflicted with advanced prostate cancer (PCa), particularly those experiencing biochemical relapse or ineligible for surgical intervention. This study serves to consolidate recent research findings on therapeutic vaccines targeting prostate tumors while delineating prevalent challenges within vaccine research and development.
Methods: We searched electronic databases, including PubMed, Web of Science, Embase, and Scopus, up to August 31, 2024, using keywords such as 'vaccine', 'prostate cancer', 'immunotherapy', and others. We reviewed studies on various therapeutic vaccines, including dendritic cell-based, antigen, nucleic acid, and tumor cell vaccines.
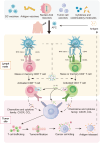
Results: Studies consistently showed that therapeutic vaccines, notably DC vaccines, had favorable safety profiles with few adverse effects. These vaccines, with varied antigenic formulations, demonstrated strong clinical outcomes, as indicated by metrics such as PSA response rates (9.5%-58%), extended PSA doubling times (52.9%-89.7%), overall survival durations (17.7-33.8 months), two-year mortality rates (0%-12.5%), biochemical relapse rates (42%-73%), and antigen-specific immune responses (33.3%-71.4% in responsive groups).
Conclusion: While clinical data for tumor vaccines have illuminated robust evidence of tumoricidal activity, the processes of their formulation and deployment are riddled with complexities. Combining vaccines with other therapies may enhance outcomes, and future research should focus on early interventions and deciphering the immune system's role in oncogenesis.
Keywords: immunotherapy; prognosis; prostate cancer; therapeutic tumor vaccine; tumor treatment.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Sung H., Ferlay J., Siegel R. L., et al., “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians 71, no. 3 (2021): 209–249. - PubMed
-
- Litwin M. S. and Tan H. J., “The Diagnosis and Treatment of Prostate Cancer: A Review,” Journal of the American Medical Association 317, no. 24 (2017): 2532–2542. - PubMed
-
- Alcorn T., “Yinghao Sun: Leader of Research on Prostate Cancer in China,” Lancet 385, no. 9965 (2015): 321. - PubMed
-
- Saxena M., van der Burg S. H., Melief C. J. M., and Bhardwaj N., “Therapeutic Cancer Vaccines,” Nature Reviews. Cancer 21, no. 6 (2021): 360–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

