Four newly synthesized enones induce mitochondrial-mediated apoptosis and G2/M cell cycle arrest in colorectal and cervical cancer cells
- PMID: 39463485
- PMCID: PMC11505670
- DOI: 10.1039/d4ra06529h
Four newly synthesized enones induce mitochondrial-mediated apoptosis and G2/M cell cycle arrest in colorectal and cervical cancer cells
Abstract
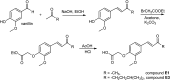
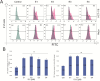
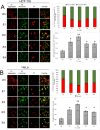
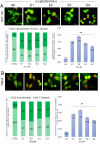
Over the last few decades, we have gained insight into how researchers attempted to modify some natural molecules to be utilized as prospective agents for cancer treatment. Many scientists synthesized new natural compounds by incorporating specific functional groups and metals that improved their antitumor activity while reducing undesirable side effects. In this investigation, we synthesized four novel structurally modified enones that differ in the functional groups attached to the carbonyl group of the enone system (methyl - E1; isopropyl - E2; isobutyl - E3; and cyclopropyl - E4) and explored their anticancer potential against human carcinoma of the colon HCT-116, the cervical HeLa, and normal lung cells MRC-5. From the findings, all the newly synthesized enones exhibited potent cytotoxic activity against the cancer cells while normal cells remained unharmed, with varying potencies among the various enones. We employed the MTT assay to assess enones's (E1-E4) cytotoxic effects, IC50 values and selectivity index in tumor cells. Furthermore, the newly synthesized enones induced cell death in cancer cells through apoptosis by promoting changes in cellular morphology, activating apoptotic regulators Bax and caspase 3, and inhibiting Bcl-2. The enones induced changes in the mitochondrial membrane potential, a release of cytochrome c, and a cell cycle arrest at the G2/M phase, thus inhibiting the growth of cancer cells. In conclusion, we demonstrated the anticancer potential of newly synthesized enones as promising candidates for future cancer treatments, especially for colon cancer, due to their selective cytotoxicity against these cancer cells. Further, in vivo studies are warranted to explore their full therapeutic potential.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
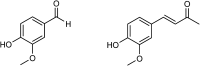






References
-
- Younis M. R., Wang C., Younis M. A. and Xia X. H., Nanobiosensors: from Design to Applications, 2020, pp. 171–201
-
- Bondar O. V. Karwt R. Mohammad T. Pavelyev R. S. Pugachev M. V. Ygaiev B. B. Kayumov A. R. Aimaletdinov A. M. Shtyrlin Y. G. Russ. J. Bioorg. Chem. 2023;49:797–814.
-
- Pavelyev R. S. Bondar O. V. Nguyen T. N. T. Ziganshina A. A. Al Farroukh M. Karwt R. Alekbaeva G. D. Pugachev M. V. Yamaleeva Z. R. Kataeva O. N. Balakin K. V. Shtyrlin Y. G. Bioorg. Med. Chem. 2018;26:5824–5837. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

