Gentle Rhodamines for Live-Cell Fluorescence Microscopy
- PMID: 39463828
- PMCID: PMC11503488
- DOI: 10.1021/acscentsci.4c00616
Gentle Rhodamines for Live-Cell Fluorescence Microscopy
Abstract
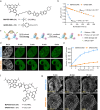
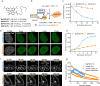
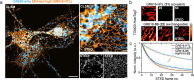
Rhodamines have been continuously optimized in brightness, biocompatibility, and color to fulfill the demands of modern bioimaging. However, the problem of phototoxicity caused by the excited fluorophore under long-term illumination has been largely neglected, hampering their use in time-lapse imaging. Here we introduce cyclooctatetraene (COT) conjugated rhodamines that span the visible spectrum and exhibit significantly reduced phototoxicity. We identified a general strategy for the generation of Gentle Rhodamines, which preserved their outstanding spectroscopic properties and cell permeability while showing an efficient reduction of singlet-oxygen formation and diminished cellular photodamage. Paradoxically, their photobleaching kinetics do not go hand in hand with reduced phototoxicity. By combining COT-conjugated spirocyclization motifs with targeting moieties, these Gentle Rhodamines compose a toolkit for time-lapse imaging of mitochondria, DNA, and actin, and synergize with covalent and exchangeable HaloTag labeling of cellular proteins with less photodamage than their commonly used precursors. Taken together, the Gentle Rhodamines generally offer alleviated phototoxicity and allow advanced video recording applications, including voltage imaging.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): K.J. and L.W. are inventors of the patent Cell-permeable fluorogenic fluorophores which was filed by the Max Planck Society, for which Spirochrome AG owns a license. Z.C., T.L., Z.Y., Y.Z, P.C., and H.Z. are inventors of a patent application protecting the compounds presented in this study which was submitted by Peking University. L.R., S.P., and K.J. own shares of Spirochrome AG. Z.C. owns shares of Genvivo tech. The remaining authors declare no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous
