Ultra-High Purity and Productivity Separation of CO2 and C2H2 from CH4 in Rigid Layered Ultramicroporous Material
- PMID: 39463839
- PMCID: PMC11503503
- DOI: 10.1021/acscentsci.4c01125
Ultra-High Purity and Productivity Separation of CO2 and C2H2 from CH4 in Rigid Layered Ultramicroporous Material
Abstract
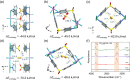
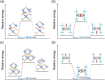
Efficiently obtaining both high-purity gas-phase and adsorbed-phase products in a single physisorption process presents the challenge of simultaneously achieving high selectivity and uptake and rapid diffusion in adsorbents. With a focus on natural gas purification and high-purity acetylene production, we report for the first time that the synergistic ligand/anion binding mode and multiple diffusion pathways in a robust 2D layered ultramicroporous framework (ZUL-100) enable unprecedented carbon dioxide/methane and acetylene/methane separation performance. Taking advantage of its rich anion, functional ligand ,and rigid 3D interpenetrated ultramicroporous channels, ZUL-100 achieved record IAST selectivities for equimolar carbon dioxide/methane (3.2 × 105) and acetylene/methane (1.7 × 1010) mixtures, accompanied by record dynamic uptakes of carbon dioxide (3.10 mmol/g) and acetylene (4.79 mmol/g), respectively. The strong affinity and fast mass transfer of carbon dioxide and acetylene on ZUL-100 were systematically elucidated by a combination of in situ FTIR, single-crystal XRD, kinetic tests, and DFT-D adsorption/diffusion modeling. In particular, high-purity (≥99.999%) methane and carbon dioxide (acetylene) can both be obtained on ZUL-100 through a single adsorption-desorption cycle, with exceptional productivity (2.81-4.22 mmol/g of methane, 2.96 mmol/g of carbon dioxide, and 4.31 mmol/g of acetylene) and high yield (95.5% for carbon dioxide and 90.0% for acetylene).
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Adu E.; Zhang Y.; Liu D. Current Situation of Carbon Dioxide Capture, Storage, and Enhanced Oil Recovery in the Oil and Gas Industry. Canadian Journal of Chemical Engineering 2019, 97 (5), 1048–1076. 10.1002/cjce.23393. - DOI
-
- Dai J.; Xie D.; Liu Y.; Zhang Z.; Yang Y.; Yang Q.; Ren Q.; Bao Z. Supramolecular Metal–Organic Framework for CO2/CH4 and CO2/N2 Separation. Ind. Eng. Chem. Res. 2020, 59 (16), 7866–7874. 10.1021/acs.iecr.0c00447. - DOI
LinkOut - more resources
Full Text Sources
