This is a preprint.
Mice with Reduced PAR4 Reactivity show Decreased Venous Thrombosis and Platelet Procoagulant Activity
- PMID: 39463946
- PMCID: PMC11507748
- DOI: 10.1101/2024.10.14.617127
Mice with Reduced PAR4 Reactivity show Decreased Venous Thrombosis and Platelet Procoagulant Activity
Update in
-
Mice with reduced protease-activated receptor 4 reactivity show decreased venous thrombosis and platelet procoagulant activity.J Thromb Haemost. 2025 Apr;23(4):1278-1288. doi: 10.1016/j.jtha.2024.12.031. Epub 2025 Jan 9. J Thromb Haemost. 2025. PMID: 39798922 Free PMC article.
Abstract
Background: Hypercoagulation and thrombin generation are major risk factors for venous thrombosis. Sustained thrombin signaling through PAR4 promotes platelet activation, phosphatidylserine exposure, and subsequent thrombin generation. A single-nucleotide polymorphism in PAR4 (rs2227376) changes proline to leucine extracellular loop 3 (P310L), which decreases PAR4 reactivity and is associated with a lower risk for venous thromboembolism (VTE) in a GWAS meta-analysis.
Objective: The goal of this study is to determine the mechanism for the association of rs2227376 with reduced risk for VTE in using mice with a homologous mutation (PAR4-P322L).
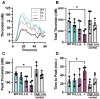
Methods: Venous thrombosis was examined using our recently generated PAR4-P322L mice in the inferior vena cava stasis and stenosis models. Coagulation and clot stability was measured using rotational thromboelastometry (ROTEM). Thrombin generating potential was measured in platelet-rich plasma. Phosphatidylserine surface expression and platelet-neutrophil aggregates were analyzed using flow cytometry.
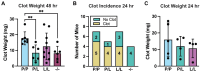
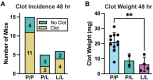
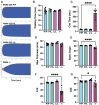
Results: PAR4P/L and PAR4L/L had reduced incidence and size of venous clots at 48 hours. PAR4P/L and PAR4L/L platelets had progressively decreased phosphatidylserine in response to thrombin and convulxin, which led to decreased thrombin generation and decreased PAR4-mediated platelet-neutrophil aggregation.
Conclusions: The leucine allele in extracellular loop 3, PAR4-322L leads to fewer procoagulant platelets and decreased endogenous thrombin potential. This decreased ability to generate thrombin offers a mechanism for PAR4's role in VTE highlighting a key role for PAR4 signaling.
Keywords: animal model; blood platelets; protease-activated receptor 4; single nucleotide polymorphisms; thrombin receptor.
Conflict of interest statement
Authors conflict of interest statement: Elizabeth A. Knauss, Johana Guci, Norman Luc, Dante Disharoon, Grace H. Huang, Anirban Sen Gupta, and Marvin T. Nieman all declare no conflicts of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
