This is a preprint.
Primary cilia shape postnatal astrocyte development through Sonic Hedgehog signaling
- PMID: 39464094
- PMCID: PMC11507945
- DOI: 10.1101/2024.10.17.618851
Primary cilia shape postnatal astrocyte development through Sonic Hedgehog signaling
Update in
-
Primary cilia shape postnatal astrocyte development through Sonic Hedgehog signaling.J Cell Sci. 2025 Oct 15;138(20):jcs263965. doi: 10.1242/jcs.263965. Epub 2025 May 19. J Cell Sci. 2025. PMID: 40110664
Abstract
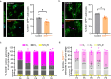
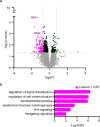
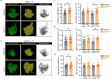
Primary cilia function as specialized signaling centers that regulate many cellular processes including neuron and glia development. Astrocytes possess cilia, but the function of cilia in astrocyte development remains largely unexplored. Critically, dysfunction of either astrocytes or cilia contributes to molecular changes observed in neurodevelopmental disorders. Here, we show that a sub-population of developing astrocytes in the prefrontal cortex are ciliated. This population corresponds to proliferating astrocytes and largely expresses the ciliary protein ARL13B. Genetic ablation of astrocyte cilia in vivo at two distinct stages of astrocyte development results in changes to Sonic Hedgehog (Shh) transcriptional targets. We show that Shh activity is decreased in immature and mature astrocytes upon loss of cilia. Furthermore, loss of cilia in immature astrocytes results in decreased astrocyte proliferation and loss of cilia in mature astrocytes causes enlarged astrocyte morphology. Together, these results indicate that astrocytes require cilia for Shh signaling throughout development and uncover functions for astrocyte cilia in regulating astrocyte proliferation and maturation. This expands our fundamental knowledge of astrocyte development and cilia function to advance our understanding of neurodevelopmental disorders.
Keywords: Sonic Hedgehog; astrocytes; cilia; development; morphology; proliferation.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
