This is a preprint.
Virulence-linked adhesin drives mutualist colonization of the bee gut via biofilm formation
- PMID: 39464101
- PMCID: PMC11507737
- DOI: 10.1101/2024.10.14.618124
Virulence-linked adhesin drives mutualist colonization of the bee gut via biofilm formation
Abstract
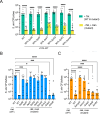
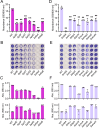
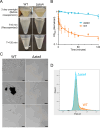
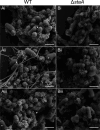
Bacterial biofilms are stable multicellular structures that can enable long term host association. Yet, the role of biofilms in supporting gut mutualism is still not fully understood. Here, we investigate Snodgrassella alvi, a beneficial bacterial symbiont of honey bees, and find that biofilm formation is required for its colonization of the bee gut. We constructed fifteen S. alvi mutants containing knockouts of genes known to promote colonization with putative roles in biofilm formation. Genes required for colonization included staA and staB, encoding trimeric autotransporter adhesins (TAAs) and mltA, encoding a lytic transglycosylase. Intriguingly, TAAs are considered virulence factors in pathogens but support mutualism by the symbiont S. alvi. In vitro, biofilm formation was reduced in ΔstaB cells and abolished in the other two mutants. Loss of staA also reduced auto-aggregation and cell-cell connections. Based on structural predictions, StaA/B are massive (>300 nm) TAAs with many repeats in their stalk regions. Further, we find that StaA/B are conserved across Snodgrassella species, suggesting that StaA/B-dependent colonization is characteristic of this symbiont lineage. Finally, staA deletion increases sensitivity to bactericidal antimicrobials, suggesting that the biofilm indirectly buffers against antibiotic stress. In all, the inability of two biofilm-deficient strains (ΔstaA and ΔmltA) to effectively mono-colonize bees indicates that S. alvi biofilm formation is required for colonization of the bee gut. We envision the bee gut system as a genetically tractable model for studying the physical basis of biofilm-mutualist-gut interactions.
Conflict of interest statement
Competing interests N.A.M. and J.E.B. have a patent (US11382989B2) on the use of engineered symbionts to improve bee health.
Figures


References
-
- Thaiss C. A., Zmora N., Levy M. & Elinav E. The microbiome and innate immunity. Nature 535, 65–74 (2016). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
