This is a preprint.
Chromatin Buffers Torsional Stress During Transcription
- PMID: 39464147
- PMCID: PMC11507789
- DOI: 10.1101/2024.10.15.618270
Chromatin Buffers Torsional Stress During Transcription
Abstract
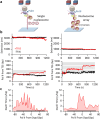
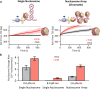
Transcription through chromatin under torsion represents a fundamental problem in biology. Pol II must overcome nucleosome obstacles and, because of the DNA helical structure, must also rotate relative to the DNA, generating torsional stress. However, there is a limited understanding of how Pol II transcribes through nucleosomes while supercoiling DNA. In this work, we developed methods to visualize Pol II rotation of DNA during transcription and determine how torsion slows down the transcription rate. We found that Pol II stalls at ± 9 pN·nm torque, nearly sufficient to melt DNA. The stalling is due to extensive backtracking, and the presence of TFIIS increases the stall torque to + 13 pN·nm, making Pol II a powerful rotary motor. This increased torsional capacity greatly enhances Pol II's ability to transcribe through a nucleosome. Intriguingly, when Pol II encounters a nucleosome, nucleosome passage becomes more efficient on a chromatin substrate than on a single-nucleosome substrate, demonstrating that chromatin efficiently buffers torsional stress via its torsional mechanical properties. Furthermore, topoisomerase II relaxation of torsional stress significantly enhances transcription, allowing Pol II to elongate through multiple nucleosomes. Our results demonstrate that chromatin greatly reduces torsional stress on transcription, revealing a novel role of chromatin beyond the more conventional view of it being just a roadblock to transcription.
Conflict of interest statement
COMPETING INTERESTS The authors declare no competing financial interests.
Figures



References
-
- Watson J.D. & Crick F.H. C. The Structure of DNA. Cold Spring Harb Sym 18, 123–131 (1953). - PubMed
-
- Watson J.D. & Crick F.H.C. Genetical Implications of the Structure of Deoxyribonucleic Acid. Nature 171, 964–967 (1953). - PubMed
-
- Killian J.L., Ma J. & Wang M.D. in RNA Polymerases as Molecular Motors: On the Road (2) 46–71 (The Royal Society of Chemistry, 2022).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
