This is a preprint.
Glucose-dependent glycosphingolipid biosynthesis fuels CD8+ T cell function and tumor control
- PMID: 39464161
- PMCID: PMC11507764
- DOI: 10.1101/2024.10.10.617261
Glucose-dependent glycosphingolipid biosynthesis fuels CD8+ T cell function and tumor control
Update in
-
Glucose-dependent glycosphingolipid biosynthesis fuels CD8+ T cell function and tumor control.Cell Metab. 2025 Sep 2;37(9):1890-1906.e11. doi: 10.1016/j.cmet.2025.07.006. Epub 2025 Aug 5. Cell Metab. 2025. PMID: 40769148
Abstract
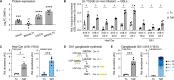
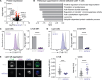
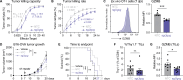
Glucose is essential for T cell proliferation and function, yet its specific metabolic roles in vivo remain poorly defined. Here, we identify glycosphingolipid (GSL) biosynthesis as a key pathway fueled by glucose that enables CD8+ T cell expansion and cytotoxic function in vivo. Using 13C-based stable isotope tracing, we demonstrate that CD8+ effector T cells use glucose to synthesize uridine diphosphate-glucose (UDP-Glc), a precursor for glycogen, glycan, and GSL biosynthesis. Inhibiting GSL production by targeting the enzymes UGP2 or UGCG impairs CD8+ T cell expansion and cytolytic activity without affecting glucose-dependent energy production. Mechanistically, we show that glucose-dependent GSL biosynthesis is required for plasma membrane lipid raft integrity and aggregation following TCR stimulation. Moreover, UGCG-deficient CD8+ T cells display reduced granzyme expression and tumor control in vivo. Together, our data establish GSL biosynthesis as a critical metabolic fate of glucose-independent of energy production-required for CD8+ T cell responses in vivo.
Keywords: CD8+ T cells; UGCG; cytotoxic function; glucose; glycosphingolipids; immunometabolism; lipid rafts; lipidomics; metabolomics; nucleotide sugar metabolism.
Conflict of interest statement
DECLARATION OF INTERESTS R.G.J. is a scientific advisor to Servier Pharmaceuticals and is a member of the Scientific Advisory Board of Immunomet Therapeutics.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
