Outcomes of treatment cessation after switching to subcutaneous vedolizumab treatment in inflammatory bowel diseases
- PMID: 39464507
- PMCID: PMC11503703
- DOI: 10.1177/17562848241290636
Outcomes of treatment cessation after switching to subcutaneous vedolizumab treatment in inflammatory bowel diseases
Abstract
Background: The usability of subcutaneous vedolizumab (s.c. VDZ) treatment in inflammatory bowel diseases (IBD; ulcerative colitis (UC), Crohn's disease (CD)) has been proven via clinical trials while real-world data collection is ongoing.
Objectives: Our study evaluates the effectiveness, safety, patients' preferences, and psychological factors associated with s.c. VDZ treatment, after switching from intravenous (i.v.) formulation.
Design: Prospective, multicenter cohort study including IBD patients switching from i.v. VDZ to s.c. treatment and were evaluated over 52 weeks.
Methods: Serum VDZ levels and C-reactive protein (CRP) were measured at the baseline and w52. At w12, a questionnaire on the patient's satisfaction and psychological characteristics was administered. The primary outcome was the drug persistence rate (cessation was due to loss of response (LOR), adverse events, patient request, and other causes) at w52, while the secondary outcomes were the changes in the clinical corticosteroid-free remission (CSFR) and biochemical remission (BR; CRP ⩽ 5 mg/L) rates, safety issues, serum drug levels, patients' preferences, and psychological features.
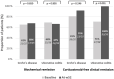
Results: In total, 70 IBD patients were evaluated (32 CD patients, 38 UC patients; male/female ratio: 41.4%; median age: 43.2 years). In the CD group, 81.3% were in CSFR and 65.6% were in BR, while in the UC group, 71.7% were in CSFR and 69.4% were in BR. Overall, 17.1% of the patients ceased s.c. VDZ treatment after a median of 26.2 (interquartile range 20-47) weeks. LOR was registered in 3/12 ceased patients. In addition, CSFR and BR rates were stable, while serum VDZ levels increased by w52 (p < 0.001).
Conclusion: The transition from i.v. to s.c. VDZ treatment was effective, the overall persistence rate was associated with high serum drug levels, and no novel safety issues were reported. Although s.c. administration after induction can save resources, some patients still insisted on i.v. VDZ treatment, due to its proven formulation.
Keywords: Crohn’s disease; IBD; patient experience; subcutaneous vedolizumab; switching; ulcerative colitis.
© The Author(s), 2024.
Conflict of interest statement
K.F. has received speaker’s honoraria from AbbVie, Janssen, Ferring, Takeda, and Goodwill Pharma. P.L.L. has been a speaker and/or advisory board member: AbbVie, Amgen, Bristol Myers Squibb, Celltrion, Fresenius Kabi, Gilead, Janssen, Eli Lilly, Merck, Organon, Pendopharm, Pfizer, Roche, Sandoz, and Takeda and has received unrestricted research grant: Gilead, Pfizer, and Takeda. T.M. has received speaker’s honoraria from MSD, AbbVie, Egis, Goodwill Pharma, Takeda, Pfizer, Janssen, Sandoz, MundiPharma, Phytotec, Roche, Fresenius, and Teva. P.B., T.R., P.S., Á.I., L.D.S., D.K., A.D., B.F., E.I., A.B., Z.S.B., A.F., R.B., Z.S.Z., W.A., and T.B. have no conflict of interest to disclose.
Figures


References
-
- Burisch J, Jess T, Martinato M, et al.. The burden of inflammatory bowel disease in Europe. J Crohns Colitis 2013; 7(4): 322–337. - PubMed
-
- Turner D, Ricciuto A, Lewis A, et al.. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021; 160(5): 1570–1583. - PubMed
-
- Sandborn WJ, Feagan BG, Rutgeerts P, et al.. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369(8): 711–721. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

