Diversity-driven, efficient exploration of a MOF design space to optimize MOF properties
- PMID: 39464600
- PMCID: PMC11499977
- DOI: 10.1039/d4sc03609c
Diversity-driven, efficient exploration of a MOF design space to optimize MOF properties
Abstract
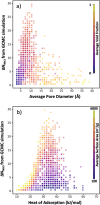
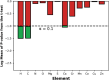
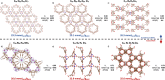
Metal-organic frameworks (MOFs) promise to engender technology-enabling properties for numerous applications. However, one significant challenge in MOF development is their overwhelmingly large design space, which is intractable to fully explore even computationally. To find diverse optimal MOF designs without exploring the full design space, we develop Vendi Bayesian optimization (VBO), a new algorithm that combines traditional Bayesian optimization with the Vendi score, a recently introduced interpretable diversity measure. Both Bayesian optimization and the Vendi score require a kernel similarity function, we therefore also introduce a novel similarity function in the space of MOFs that accounts for both chemical and structural features. This new similarity metric enables VBO to find optimal MOFs with properties that may depend on both chemistry and structure. We statistically assessed VBO by its ability to optimize three NH3-adsorption dependent performance metrics that depend, to different degrees, on MOF chemistry and structure. With ten simulated campaigns done for each metric, VBO consistently outperformed random search to find high-performing designs within a 1000-MOF subset for (i) NH3 storage, (ii) NH3 removal from membrane plasma reactors, and (iii) NH3 capture from air. Then, with one campaign dedicated to finding optimal MOFs for NH3 storage in a "hybrid" ∼10 000-MOF database, we identify twelve extant and eight hypothesized MOF designs with potentially record-breaking working capacity ΔN NH3 between 300 K and 400 K at 1 bar. Specifically, the best MOF designs are predicted to (i) achieve ΔN NH3 values between 23.6 and 29.3 mmol g-1, potentially surpassing those that MOFs previously experimentally tested for NH3 adsorption would have at the proposed operation conditions, (ii) be thermally stable at the operation conditions and (iii) require only ca. 10% of the energy content in NH3 to release the stored molecule from the MOF. Finally, the analysis of the generated simulation data during the search indicates that a pore size of around 10 Å, a heat of adsorption around 33 kJ mol-1, and the presence of Ca could be part of MOF design rules that could help optimize NH3 working capacity at the proposed operation conditions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Gomez-Gualdron D. A. Gutov O. V. Krungleviciute V. Borah B. Mondloch J. E. Hupp J. T. Yildirim T. Farha O. K. Snurr R. Q. Computational design of metal–organic frameworks based on stable zirconium building units for storage and delivery of methane. Chem. Mater. 2014;26:5632–5639. doi: 10.1021/cm502304e. - DOI
-
- Gómez-Gualdrón D. A. Colón Y. J. Zhang X. Wang T. C. Chen Y.-S. Hupp J. T. Yildirim T. Farha O. K. Zhang J. Snurr R. Q. Evaluating topologically diverse metal–organic frameworks for cryo-adsorbed hydrogen storage. Energy Environ. Sci. 2016;9:3279–3289. doi: 10.1039/C6EE02104B. - DOI
LinkOut - more resources
Full Text Sources

