A cocktail of Cu2+- and Zn2+-peptoid-based chelators can stop ROS formation for Alzheimer's disease therapy
- PMID: 39464602
- PMCID: PMC11503657
- DOI: 10.1039/d4sc04313h
A cocktail of Cu2+- and Zn2+-peptoid-based chelators can stop ROS formation for Alzheimer's disease therapy
Abstract
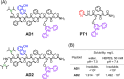
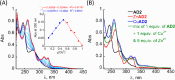
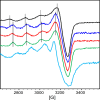
The formation of reactive oxygen species (ROS) in the brain is a major cause of neuropathologic degradation associated with Alzheimer's Disease (AD). It has been suggested that the copper (Cu)-amyloid-β (Aβ) peptide complex can lead to ROS formation in the brain. An external chelator for Cu that can extract Cu from the CuAβ complex should inhibit the formation of ROS, making Cu chelation an excellent therapeutic approach for AD. Such a chelator should possess high selectivity for Cu over zinc (Zn), which is also present within the synaptic cleft. However, such selectivity is generally hard to achieve in one molecule due to the similarities in the binding preferences of these two metal ions. As an alternative to monotherapy (where Cu extraction is performed using a single chelator), herein we describe a variation of combination therapy - a novel cocktail approach, which is based on the co-administration of two structurally different peptidomimetic chelators, aiming to target both Cu2+ and Zn2+ ions simultaneously but independently from each other. Based on rigorous spectroscopic experiments, we demonstrate that our peptidomimetic cocktail allows, for the first time, the complete and immediate inhibition of ROS production by the CuAβ complex in the presence of Zn2+. In addition, we further demonstrate the high stability of the cocktail under simulated physiological conditions and its resistance to proteolytic degradation by trypsin and report the water/n-octanol partition coefficient, initially assessing the blood-brain barrier (BBB) permeability potential of the chelators.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
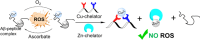

References
LinkOut - more resources
Full Text Sources
Research Materials

