Leveraging ordered voids in microporous perovskites for intercalation and post-synthetic modification
- PMID: 39464608
- PMCID: PMC11497115
- DOI: 10.1039/d4sc04378b
Leveraging ordered voids in microporous perovskites for intercalation and post-synthetic modification
Abstract
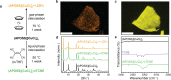
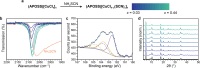
We report the use of porous organic layers in two-dimensional hybrid organic-inorganic perovskites (HOIPs) to facilitate permanent small molecule intercalation and new post-synthetic modifications. While HOIPs are well-studied for a variety of optoelectronic applications, the ability to manipulate their structure after synthesis is another handle for control of physical properties and could even enable use in future applications. If designed properly, a porous interlayer could facilitate these post-synthetic transformations. We show that for a series of copper-halide perovskites, a crystalline arrangement of designer ammonium groups allows for permanently porous interlayer space to be accessed at room temperature. Intercalation of the electroactive molecules ferrocene and tetracyanoethylene into this void space can be performed with tunable loadings, and these intercalated perovskites are stable for months. The porosity also enables reactivity at the copper-halide layer, allowing for facile halide replacement. Through this, we access previously unobserved reactivity with halogens to perform halide substitution, and even replace halides with pseudohalides. In the latter case, the porous structure allows for stabilization of new phases, specifically a novel copper-thiocyanate perovskite phase, only accessible through post-synthetic modification. We envision that this broad design strategy can be expanded to other industrially relevant HOIPs to create a new class of highly adjustable perovskites.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Smith M. D. Crace E. J. Jaffe A. Karunadasa H. I. Annu. Rev. Mater. Res. 2018;48:111–136. doi: 10.1146/annurev-matsci-070317-124406. - DOI
LinkOut - more resources
Full Text Sources

