Lung cancer cell-derived exosomes: progress on pivotal role and its application in diagnostic and therapeutic potential
- PMID: 39464709
- PMCID: PMC11502357
- DOI: 10.3389/fonc.2024.1459178
Lung cancer cell-derived exosomes: progress on pivotal role and its application in diagnostic and therapeutic potential
Abstract
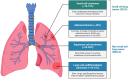
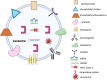
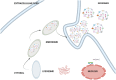
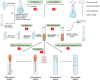
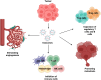
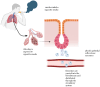
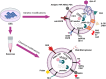
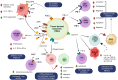
Lung cancer is frequently detected in an advanced stage and has an unfavourable prognosis. Conventional therapies are ineffective for the treatment of metastatic lung cancer. While certain molecular targets have been identified as having a positive response, the absence of appropriate drug carriers prevents their effective utilization. Lung cancer cell-derived exosomes (LCCDEs) have gained attention for their involvement in the development of cancer, as well as their potential for use in diagnosing, treating, and predicting the outcome of lung cancer. This is due to their biological roles and their inherent ability to transport biomolecules from the donor cells. Lung cancer-associated cell-derived extracellular vesicles (LCCDEVs) have the ability to enhance cell proliferation and metastasis, influence angiogenesis, regulate immune responses against tumours during the development of lung cancer, control drug resistance in lung cancer treatment, and are increasingly recognised as a crucial element in liquid biopsy evaluations for the detection of lung cancer. Therapeutic exosomes, which possess inherent intercellular communication capabilities, are increasingly recognised as effective vehicles for targeted drug delivery in precision medicine for tumours. This is due to their exceptional biocompatibility, minimal immunogenicity, low toxicity, prolonged circulation in the bloodstream, biodegradability, and ability to traverse different biological barriers. Currently, multiple studies are being conducted to create new means of diagnosing and predicting outcomes using LCCDEs, as well as to develop techniques for utilizing exosomes as effective carriers for medication delivery. This paper provides an overview of the current state of lung cancer and the wide range of applications of LCCDEs. The encouraging findings and technologies suggest that the utilization of LCCDEs holds promise for the clinical treatment of lung cancer patients.
Keywords: diagnostic; exosome; lung cancer; lung cancer cell-derived exosomes; therapeutic potential.
Copyright © 2024 Abdul Manap, Ngwenya, Kalai Selvan, Arni, Hassan, Mohd Rudy and Abdul Razak.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

