Cardiomyopathy: pathogenesis and therapeutic interventions
- PMID: 39465141
- PMCID: PMC11502724
- DOI: 10.1002/mco2.772
Cardiomyopathy: pathogenesis and therapeutic interventions
Abstract
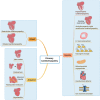
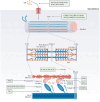
Cardiomyopathy is a group of disease characterized by structural and functional damage to the myocardium. The etiologies of cardiomyopathies are diverse, spanning from genetic mutations impacting fundamental myocardial functions to systemic disorders that result in widespread cardiac damage. Many specific gene mutations cause primary cardiomyopathy. Environmental factors and metabolic disorders may also lead to the occurrence of cardiomyopathy. This review provides an in-depth analysis of the current understanding of the pathogenesis of various cardiomyopathies, highlighting the molecular and cellular mechanisms that contribute to their development and progression. The current therapeutic interventions for cardiomyopathies range from pharmacological interventions to mechanical support and heart transplantation. Gene therapy and cell therapy, propelled by ongoing advancements in overarching strategies and methodologies, has also emerged as a pivotal clinical intervention for a variety of diseases. The increasing number of causal gene of cardiomyopathies have been identified in recent studies. Therefore, gene therapy targeting causal genes holds promise in offering therapeutic advantages to individuals diagnosed with cardiomyopathies. Acting as a more precise approach to gene therapy, they are gradually emerging as a substitute for traditional gene therapy. This article reviews pathogenesis and therapeutic interventions for different cardiomyopathies.
Keywords: cardiomyopathy; disease‐causing gene; gene therapy; pathogenesis; personalized medicine; therapeutic interventions.
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Lipshultz SE, Law YM, Asante‐Korang A, et al. Cardiomyopathy in Children: Classification and Diagnosis: A Scientific Statement From the American Heart Association. Circulation. 2019;140(1):e9‐e68. - PubMed
-
- Dadson K, Hauck L, Billia F. Molecular mechanisms in cardiomyopathy. Clin Sci (Lond). 2017;131(13):1375‐1392. - PubMed
-
- Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807‐1816. - PubMed
-
- Strong A, Musunuru K. Genome editing in cardiovascular diseases. Nat Rev Cardiol. 2017;14(1):11‐20. - PubMed
-
- Tan K, Foo R, Loh M. Cardiomyopathy in Asian Cohorts: Genetic and Epigenetic Insights. Circ Genom Precis Med. 2023;16(5):496‐506. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
