Targeting the VEGFR2 signaling pathway for angiogenesis and fibrosis regulation in neovascular age-related macular degeneration
- PMID: 39465270
- PMCID: PMC11514265
- DOI: 10.1038/s41598-024-76258-4
Targeting the VEGFR2 signaling pathway for angiogenesis and fibrosis regulation in neovascular age-related macular degeneration
Abstract
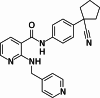
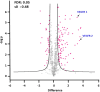
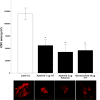
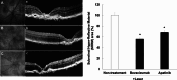
Neovascular age-related macular degeneration (nAMD) is characterized by abnormal blood vessel growth from the choroid, leading to complications and eventual blindness. Despite anti-VEGF therapy, subretinal fibrosis remains a major concern, as VEGF/VEGF receptor-2 (VEGFR2) signaling can contribute to both angiogenesis and fibrosis. For the identification of the aqueous humor proteome, we performed liquid chromatography with tandem mass spectrometry analysis. To investigate the potential therapeutic effects of targeting the VEGF signaling pathway using apatinib, a highly selective VEGFR2 tyrosine kinase inhibitor, this study employed in vitro (THP-1 conditioned media-treated ARPE-19 cells) and in vivo (laser-induced choroidal neovascularization mouse) models of nAMD. This study revealed elevated VEGFR2 protein levels in the aqueous humor of nAMD patients, suggesting a potential target to mitigate neovascularization and fibrosis in nAMD. Apatinib effectively reduced VEGFA and αSMA levels in both in vitro and in vivo models. Moreover, apatinib showed improvement in laser-induced subretinal hyper-reflective lesions. The action mechanism was linked to the inhibition of VEGFR2 activation, leading to the suppression of both angiogenesis and fibrosis through the downregulation of STAT3 phosphorylation. Therefore, the VEGFR2 signaling pathway appears to play a central role in the development of nAMD by regulating both angiogenesis and fibrosis.
Keywords: Apatinib; Choroidal neovascularization; Fibrosis; Neovascular age-related macular degeneration; Tyrosine kinase inhibitor; Vascular endothelial growth factor receptor-2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Witmer, A. N., Vrensen, G. F., Van Noorden, C. J. & Schlingemann, R. O. Vascular endothelial growth factors and angiogenesis in eye disease. Prog. Retin. Eye Res.22, 1–29. 10.1016/s1350-9462(02)00043-5 (2003). - PubMed
-
- Heier, J. S. et al. Ranibizumab for treatment of neovascular age-related macular degeneration: A phase I/II multicenter, controlled, multidose study. Ophthalmology113(633), e631-634. 10.1016/j.ophtha.2005.10.052 (2006). - PubMed
-
- Rosenfeld, P. J. et al. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med.355, 1419–1431. 10.1056/NEJMoa054481 (2006). - PubMed
-
- Heier, J. S. et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology119, 2537–2548. 10.1016/j.ophtha.2012.09.006 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

