Performance of the Flash10 COVID-19 point-of-care molecular test
- PMID: 39465327
- PMCID: PMC11514266
- DOI: 10.1038/s41598-024-77837-1
Performance of the Flash10 COVID-19 point-of-care molecular test
Abstract
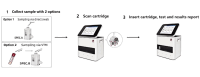
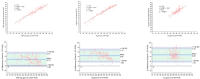
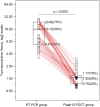
After the COVID-19 pandemic, fever clinics urgently require rapid nucleic acid tests to enhance their capacity for timely pathogen detection. This study evaluated the analytical performance and clinical utility of the Flash10 SARS-CoV-2 point-of-care test (Flash10 POCT) for detecting SARS-CoV-2 in patients with fever in the adult fever clinic in Beijing Tsinghua Changgung Hospital from August 1 to August 30, 2023. The analytical performance and clinical utility of the Flash10 POCT for detecting SARS-CoV-2 were assessed in 125 patients with fever syndrome in the adult fever clinic. The Flash10 POCT demonstrated an analytical precision of 3.1% for the Ct values of the ORF1ab gene and 2.9% for the Ct values of the N gene in SARS-CoV-2 nucleic acid testing. Furthermore, the Flash10 POCT demonstrated a lower limit of detection (LoD) of 100 copies/mL, with no detected aerosol contamination leakage. Of the 125 patients (median age 61.9 years, 52% male and 48% female), both the Flash10 POCT and RT-PCR tests yielded positive results for 100 patients and negative results for 25 patients (Fisher's exact test, p < 0.0001). The median turn-around-time for the Flash10 POCT was significantly shorter, at 1.05 h, compared to 16.15 h required for RT-PCR tests (Wilcoxon signed rank test, p < 0.0001). The Flash10 POCT showed high analytical performance, achieving a 100% detection rate for SARS-CoV-2 compared to RT-PCR tests, while also exhibiting a significantly shorter turn-around-time. Implementing the Flash10 POCT had the potential to expedite the care of adults presenting with fever.
Keywords: COVID-19; SARS-CoV-2; diagnostic; point-of-care testing.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
[Analytical performance evaluation of Flash10 point-of-care testing in severe acute respiratory syndrome coronavirus 2 nucleic acid detection].Zhonghua Yi Xue Za Zhi. 2024 Dec 17;104(47):4323-4329. doi: 10.3760/cma.j.cn112137-20240719-01661. Zhonghua Yi Xue Za Zhi. 2024. PMID: 39667770 Chinese.
-
Evaluation of a new point-of-care quantitative reverse transcription polymerase chain test for detecting severe acute respiratory syndrome coronavirus 2.J Clin Lab Anal. 2021 Oct;35(10):e23992. doi: 10.1002/jcla.23992. Epub 2021 Sep 14. J Clin Lab Anal. 2021. PMID: 34519100 Free PMC article.
-
Multisite Clinical Validation of Isothermal Amplification-Based SARS-CoV-2 Detection Assays Using Different Sampling Strategies.Microbiol Spectr. 2021 Oct 31;9(2):e0084621. doi: 10.1128/Spectrum.00846-21. Epub 2021 Oct 20. Microbiol Spectr. 2021. PMID: 34668736 Free PMC article.
-
Point of care molecular and antigen detection tests for COVID-19: current status and future prospects.Expert Rev Mol Diagn. 2022 Aug;22(8):797-809. doi: 10.1080/14737159.2022.2122712. Epub 2022 Sep 14. Expert Rev Mol Diagn. 2022. PMID: 36093682 Review.
-
Machine learning in point-of-care testing: innovations, challenges, and opportunities.Nat Commun. 2025 Apr 2;16(1):3165. doi: 10.1038/s41467-025-58527-6. Nat Commun. 2025. PMID: 40175414 Free PMC article. Review.
References
-
- Schwartz, R. A. & Kapila, R. Pandemics throughout the centuries. Clin. Dermatol.39 (1), 5–8. 10.1016/j.clindermatol.2020.12.006 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

