Endolymphatic hydrops and cochlear synaptopathy after noise exposure are distinct sequelae of hair cell stereociliary bundle trauma
- PMID: 39465341
- PMCID: PMC11514180
- DOI: 10.1038/s41598-024-77154-7
Endolymphatic hydrops and cochlear synaptopathy after noise exposure are distinct sequelae of hair cell stereociliary bundle trauma
Abstract
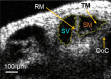
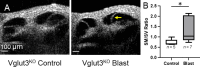
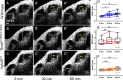
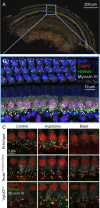
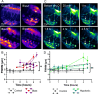
Endolymphatic hydrops, increased endolymphatic fluid within the cochlea, is the key pathologic finding in patients with Meniere's disease, a disease of episodic vertigo, fluctuating hearing loss, tinnitus, and aural fullness. Endolymphatic hydrops also can occur after noise trauma and its presence correlates with cochlear synaptopathy, a form of hearing loss caused by reduced numbers of synapses between hair cells and auditory nerve fibers. Here we tested whether there is a mechanistic link between these two phenomena by using multimodal imaging techniques to analyze the cochleae of transgenic mice exposed to blast and osmotic challenge. In vivo cochlear imaging after blast exposure revealed dynamic increases in endolymph that involved hair cell mechanoelectrical transduction channel block but not the synaptic release of glutamate at the hair cell-auditory nerve synapse. In contrast, ex vivo and in vivo auditory nerve imaging revealed that synaptopathy requires glutamate release from hair cells but not endolymphatic hydrops. Thus, although endolymphatic hydrops and cochlear synaptopathy are both observed after noise exposure, one does not cause the other. They are simply co-existent sequelae that derive from the traumatic stimulation of hair cell stereociliary bundles. Importantly, these data argue that Meniere's disease derives from hair cell transduction channel blockade.
Keywords: Auditory nerve; Cochlea; Hair cell; Hearing; Optical coherence tomography; Osmosis.
© 2024. The Author(s).
Conflict of interest statement
John Oghalai and Brian Applegate are founders of AO technologies, with the goal of translating inner ear imaging technologies for clinical purposes. The other authors have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

