The Role of GABA Receptors in Anesthesia and Sedation: An Updated Review
- PMID: 39465449
- PMCID: PMC11695389
- DOI: 10.1007/s40263-024-01128-6
The Role of GABA Receptors in Anesthesia and Sedation: An Updated Review
Abstract
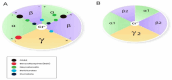
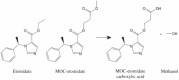
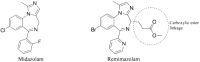
GABA (γ-aminobutyric acid) receptors are constituents of many inhibitory synapses within the central nervous system. They are formed by 5 subunits out of 19 various subunits: α1-6, β1-3, γ1-3, δ, ε, θ, π, and ρ1-3. Two main subtypes of GABA receptors have been identified, namely GABAA and GABAB. The GABAA receptor (GABAAR) is formed by a variety of combinations of five subunits, although both α and β subunits must be included to produce a GABA-gated ion channel. Other subunits are γ, δ, ε, π, and ϴ. GABAAR has many isoforms, that dictate, among other properties, their differing affinities and conductance. Drugs acting on GABAAR form the cornerstone of anesthesia and sedation practice. Some such GABAAR agonists used in anesthesia practice are propofol, etomidate, methohexital, thiopental, isoflurane, sevoflurane, and desflurane. Ketamine, nitrous oxide, and xenon are not GABAR agonists and instead inhibit glutamate receptors-mainly NMDA receptors. Inspite of its many drawbacks such as pain in injection, quick and uncontrolled conversion from sedation to general anesthesia and dose-related cardiovascular depression, propofol remains the most popular GABAR agonist employed by anesthesia providers. In addition, being formulated in a lipid emulsion, contamination and bacterial growth is possible. Literature is rife with newer propofol formulations, aiming to address many of these drawbacks, and with some degree of success. A nonemulsion propofol formulation has been developed with cyclodextrins, which form inclusion complexes with drugs having lipophilic properties while maintaining aqueous solubility. Inhalational anesthetics are also GABA agonists. The binding sites are primarily located within α+/β- and β+/α- subunit interfaces, with residues in the α+/γ- interface. Isoflurane and sevoflurane might have slightly different binding sites providing unexpected degree of selectivity. Methoxyflurane has made a comeback in Europe for rapid provision of analgesia in the emergency departments. Penthrox (Galen, UK) is the special device designed for its administration. With better understanding of pharmacology of GABAAR agonists, newer sedative agents have been developed, which utilize "soft pharmacology," a term pertaining to agents that are rapidly metabolized into inactive metabolites after producing desired therapeutic effect(s). These newer "soft" GABAAR agonists have many properties of ideal sedative agents, as they can offer well-controlled, titratable activity and ultrashort action. Remimazolam, a modified midazolam and methoxycarbonyl-etomidate (MOC-etomidate), an ultrashort-acting etomidate analog are two such examples. Cyclopropyl methoxycarbonyl metomidate is another second-generation soft etomidate analog that has a greater potency and longer half-life than MOC-etomidate. Additionally, it might not cause adrenal axis suppression. Carboetomidate is another soft analog of etomidate with low affinity for 11β-hydroxylase and is, therefore, unlikely to have clinically significant adrenocortical suppressant effects. Alphaxalone, a GABAAR agonist, is recently formulated in combination with 7-sulfobutylether-β-cyclodextrin (SBECD), which has a low hypersensitivity profile.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Funding: No funding was received for the preparation or publication of this manuscript. Conflicts of Interest: The authors declare that they have no conflicts of interest that might be relevant to the contents of this manuscript. Ethics Approval: Not applicable. Consent to Participate/Publish: Not applicable. Availability of Data and Materials: Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study. Code Availability: Not applicable. Author Contributions: A.B.P. and J.B. were involved in updating the manuscript, while B.G. provided the guidelines and reviewed the manuscript.
Figures
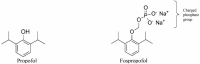
References
-
- Simeone TA, Donevan SD, Rho JM. Molecular biology and ontogeny of gamma-aminobutyric acid (GABA) receptors in the mammalian central nervous system. J Child Neurol. 2003;18:39–48 (discussion 49). - PubMed
-
- Korpi ER, Sinkkonen ST. GABA(A) receptor subtypes as targets for neuropsychiatric drug development. Pharmacol Ther. 2006;109:12–32. - PubMed
-
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–67. - PubMed
-
- Nutt D. GABAA receptors: subtypes, regional distribution, and function. J Clin Sleep Med. 2006;2(2):S7-11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

