Long-term safety of mepolizumab for up to ∼10 years in patients with severe asthma: open-label extension study
- PMID: 39465531
- PMCID: PMC11520089
- DOI: 10.1080/07853890.2024.2417184
Long-term safety of mepolizumab for up to ∼10 years in patients with severe asthma: open-label extension study
Abstract
Objectives: Long-term safety monitoring of mepolizumab is necessary to support real-world use for the treatment of severe asthma. This Long-Term Access Program assessed the safety and benefit:risk of mepolizumab in pediatric, adolescent, and adult patients with severe asthma.
Materials and methods: This was a multicenter, Phase IIIb safety, open-label extension study of multiple prior studies assessing mepolizumab in addition to standard of care (Aug 2015 - Aug 2022). Adults/adolescents (≥12 years of age) received mepolizumab 100 mg subcutaneously (SC) every 4 weeks until mepolizumab was commercialized. Pediatric patients (6-11 years of age) received mepolizumab 40 mg or 100 mg SC (bodyweight <40 or ≥40 kg, respectively) every 4 weeks. Safety was assessed every 4 weeks and benefit:risk every 12 weeks.
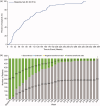
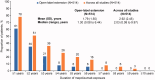
Results: Of the 514 patients enrolled, 57% were female and the mean age was 51.1 (standard deviation: 14.9) years; 24 (5%) patients were 6-17 years of age. Total cumulative mepolizumab exposure across all mepolizumab studies included in this analysis was 1500.59 patient-years; median exposure was 2.03 (range, 0.08 to 9.97) years. Overall, 37 (7%) patients experienced on-treatment serious adverse events (SAEs): 34/502 (7%) in the 100 mg SC group and 3/7 (43%) in the 40 mg SC pediatric group. Two patients experienced SAEs considered to be treatment-related by the investigator. Infections were the most common SAEs of special interest (9 [2%] patients). Physician-assessed benefit:risk of mepolizumab supported continued treatment over the study period.
Conclusions: This long-term safety analysis of mepolizumab was consistent with previous reports, with no emerging safety concerns; most patients had a favorable benefit:risk up to ∼10 years.
Clinical trial identifier: NCT00244686 (GSK ID 201956).
Keywords: Long-term access program; mepolizumab; open-label extension; safety; severe asthma with an eosinophilic phenotype.
Conflict of interest statement
Figures
References
-
- GBD Chronic Respiratory Disease Collaborators . Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. - PMC - PubMed
-
- Roche N, Garcia G, de Larrard A, et al. . Real-life impact of uncontrolled severe asthma on mortality and healthcare use in adolescents and adults: findings from the retrospective, observational RESONANCE study in France. BMJ Open. 2022;12(8):e060160. doi: 10.1136/bmjopen-2021-060160. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
