Phase I Study of ROR1-Specific CAR-T Cells in Advanced Hematopoietic and Epithelial Malignancies
- PMID: 39466024
- PMCID: PMC11788652
- DOI: 10.1158/1078-0432.CCR-24-2172
Phase I Study of ROR1-Specific CAR-T Cells in Advanced Hematopoietic and Epithelial Malignancies
Abstract
Purpose: The receptor tyrosine kinase-like orphan receptor 1 (ROR1) is expressed in hematopoietic and epithelial cancers but has limited expression on normal adult tissues. This phase I study evaluated the safety of targeting ROR1 with autologous T lymphocytes engineered to express a ROR1 chimeric antigen receptor (CAR). Secondary objectives evaluated the persistence, trafficking, and antitumor activity of CAR-T cells.
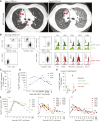
Patients and methods: Twenty-one patients with ROR1+ tumors received CAR-T cells at one of four dose levels: 3.3 × 105, 1 × 106, 3.3 × 106, and 1 × 107 cells/kg body weight, administered after lymphodepletion with cyclophosphamide/fludarabine or oxaliplatin/cyclophosphamide. Cohort A included patients with chronic lymphocytic leukemia (CLL, n = 3); cohort B included patients with triple-negative breast cancer (TNBC, n = 10) or non-small cell lung cancer (NSCLC, n = 8). A second infusion was administered to one patient in cohort A with residual CLL in the marrow and three patients in cohort B with stable disease after first infusion.
Results: Treatment was well tolerated, apart from one dose-limiting toxicity at dose level 4 in a patient with advanced NSCLC. Two of the three (67%) patients with CLL showed robust CAR-T-cell expansion and a rapid antitumor response. In patients with NSCLC and TNBC, CAR-T cells expanded to variable levels and infiltrated tumors poorly and 1 of 18 patients (5.5%) achieved partial response by RECIST 1.1.
Conclusions: ROR1 CAR-T cells were well tolerated in most patients. Antitumor activity was observed in CLL but was limited in TNBC and NSCLC. Immunogenicity of the CAR and lack of sustained tumor infiltration were identified as limitations. See related commentary by Kobold, p. 437.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
J.M. Specht reports grants from Juno Therapeutics during the conduct of the study as well as grants and personal fees from A2 Biotherapeutics; personal fees from Scripps Research Institution, GE Healthcare, Boehringer Ingelheim, Sensei Biotherapeutics, and Volastra, and grants from Lyell Immunopharma, Carisma Therapeutics, Celcuity, Genentech, Xencor, Seagen, Pfizer, and Merck outside the submitted work. In addition, J.M. Specht reports a patent for ROR1 CAR issued, licensed, and with royalties paid from Lyell Immunopharma. C.C.S. Yeung reports personal fees from TwinStrand outside the submitted work. S.M. Lee reports other support from Iovance, Lyell, Seagen, and Juno Therapeutics outside the submitted work. E.W. Newell reports personal fees and other support from ImmunoScape, Neogene, and Trojan Bio and personal fees from InduPro outside the submitted work. D.G. Maloney reports grants and personal fees from Bristol Myers Squibb/Juno Therapeutics during the conduct of the study as well as personal fees and other support from A2 Biotherapeutics and personal fees from Bristol Myers Squibb, Juno Therapeutics, and Lyell outside the submitted work. In addition, D.G. Maloney reports a patent for Fred Hutchinson Cancer Center pending and with royalties paid from Bristol Myers Squibb. S.R. Riddell reports grants from Juno Therapeutics, a Bristol Myers Squibb company, during the conduct of the study as well as grants from Bristol Myers Squibb; grants, personal fees, and other support from Lyell Immunopharma; other support from Ozette Technologies; personal fees and other support from Outpace Bio; and personal fees from Adaptive Biotechnologies and Nohla Therapeutics outside the submitted work. In addition, S.R. Riddell reports a patent for PCT/US2018/049812 pending to Lyell Immunopharma. No disclosures were reported by the other authors.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

