Mobile Stroke Unit Management in Patients With Acute Ischemic Stroke Eligible for Intravenous Thrombolysis
- PMID: 39466286
- PMCID: PMC11581552
- DOI: 10.1001/jamaneurol.2024.3659
Mobile Stroke Unit Management in Patients With Acute Ischemic Stroke Eligible for Intravenous Thrombolysis
Abstract
Importance: Clinical trials have suggested that prehospital management in a mobile stroke unit (MSU) improves functional outcomes in patients with acute ischemic stroke who are potentially eligible for intravenous thrombolysis, but there is a paucity of real-world evidence from routine clinical practice on this topic.
Objective: To determine the association between prehospital management in an MSU vs standard emergency medical services (EMS) management and the level of global disability at hospital discharge.
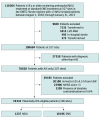
Design, setting, and participants: This was a retrospective, observational, cohort study that included consecutive patients with a final diagnosis of ischemic stroke who received either prehospital management in an MSU or standard EMS management between August 1, 2018, and January 31, 2023. Follow-up ended at hospital discharge. The primary analytic cohort included those who were potentially eligible for IV thrombolysis. A separate, overlapping cohort including all patients regardless of diagnosis was also analyzed. Patient data were obtained from the American Heart Association's Get With The Guidelines-Stroke (GWTG-Stroke) Program, a nationwide, multicenter quality assurance registry. This analysis was completed in May 2024.
Exposure: Prehospital management in an MSU (vs standard EMS management).
Main outcomes and measures: The primary efficacy end point was the utility-weighted modified Rankin Scale (UW-mRS) score. The secondary efficacy end point was independent ambulation status. The coprimary safety end points were symptomatic intracranial hemorrhage (sICH) and in-hospital mortality.
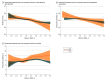
Results: Of 19 433 patients (median [IQR] age, 73 [62-83] years; 9867 female [50.8%]) treated at 106 hospitals, 1237 (6.4%) received prehospital management in an MSU. Prehospital management in an MSU was associated with a better score on the UW-mRS at discharge (adjusted mean difference, 0.03; 95% CI, 0.01-0.05) and a higher likelihood of independent ambulation at discharge (53.3% [468 of 878 patients] vs 48.3% [5868 of 12 148 patients]; adjusted risk ratio [aRR], 1.08; 95% CI, 1.03-1.13). There was no statistically significant difference in sICH (5.2% [57 of 1094] vs 4.2% [545 of 13 014]; aRR, 1.30; 95% CI, 0.94-1.75]) or in-hospital mortality (5.7% [70 of 1237] vs 6.2% [1121 of 18 196]; aRR, 1.03; 95% CI, 0.78-1.27) between the 2 groups.
Conclusions and relevance: Among patients with acute ischemic stroke potentially eligible for intravenous thrombolysis, prehospital management in an MSU compared with standard EMS management was associated with a significantly lower level of global disability at hospital discharge. These findings support policy efforts to expand access to prehospital MSU management.
Conflict of interest statement
Figures
Comment in
-
Mobile Stroke Units-Time for Legislation and Remuneration.JAMA Neurol. 2024 Dec 1;81(12):1247-1249. doi: 10.1001/jamaneurol.2024.3627. JAMA Neurol. 2024. PMID: 39466256 No abstract available.
References
-
- Lees KR, Bluhmki E, von Kummer R, et al. ; ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group . Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695-1703. doi: 10.1016/S0140-6736(10)60491-6 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

