Regional response to light illuminance across the human hypothalamus
- PMID: 39466317
- PMCID: PMC11517251
- DOI: 10.7554/eLife.96576
Regional response to light illuminance across the human hypothalamus
Abstract
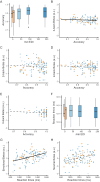
Light exerts multiple non-image-forming biological effects on physiology including the stimulation of alertness and cognition. However, the subcortical circuitry underlying the stimulating impact of light is not established in humans. We used 7 Tesla functional magnetic resonance imaging to assess the impact of variations in light illuminance on the regional activity of the hypothalamus while healthy young adults (N=26; 16 women; 24.3±2.9 y) were completing two auditory cognitive tasks. We find that, during both the executive and emotional tasks, higher illuminance triggered an activity increase over the posterior part of the hypothalamus, which includes part of the tuberomamillary nucleus and the posterior part of the lateral hypothalamus. In contrast, increasing illuminance evoked a decrease in activity over the anterior and ventral parts of the hypothalamus, encompassing notably the suprachiasmatic nucleus and another part of the tuberomammillary nucleus. Critically, the performance of the executive task was improved under higher illuminance and was negatively correlated with the activity of the posterior hypothalamus area. These findings reveal the distinct local dynamics of different hypothalamus regions that underlie the impact of light on cognition.
Keywords: 7T fMRI; circadian rhythm; cognition; human; hypothalamus; light; melanopsin; neuroscience.
© 2024, Campbell, Sharifpour et al.
Conflict of interest statement
IC, RS, JB, EB, IP, AB, EK, NM, JR, MZ, PT, FC, SS, CP, LL, GV No competing interests declared
Figures



Update of
- doi: 10.1101/2023.12.19.572317
- doi: 10.7554/eLife.96576.1
- doi: 10.7554/eLife.96576.2
References
MeSH terms
Grants and funding
- J.0222.20/Fonds De La Recherche Scientifique - FNRS Belgium
- 10.3030/860613/Marie Sklodowska-Curie Actions
- LIGHT-CABIN/ULiège-Valeo Innovation Chair "Health and Well-Being in Transport" and Sanfran
- Biomed Hub/European Regional Development Fund
- SCAIFIELD / R.8011.21/JPND - EU Joint-Programme Neurodegenerative Disease Research
LinkOut - more resources
Full Text Sources
Medical

