GABAergic Neurons in the Central Amygdala Promote Emergence from Isoflurane Anesthesia in Mice
- PMID: 39466630
- PMCID: PMC11723501
- DOI: 10.1097/ALN.0000000000005279
GABAergic Neurons in the Central Amygdala Promote Emergence from Isoflurane Anesthesia in Mice
Abstract
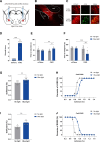
Background: Recent evidence indicates that general anesthesia and sleep-wake behavior share some overlapping neural substrates. γ-Aminobutyric acid-mediated (GABAergic) neurons in the central amygdala have a high firing rate during wakefulness and play a role in regulating arousal-related behaviors. The objective of this study was to investigate whether central amygdala GABAergic neurons participate in the regulation of isoflurane general anesthesia and uncover the underlying neural circuitry.
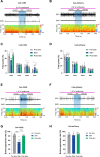
Methods: Fiber photometry recording was used to determine the changes in calcium signals of central amygdala GABAergic neurons during isoflurane anesthesia in Vgat-Cre mice. Chemogenetic and optogenetic approaches were used to manipulate the activity of central amygdala GABAergic neurons, and a righting reflex test was used to determine the induction and emergence from isoflurane anesthesia. Cortical electroencephalogram (EEG) recording was used to assess the changes in EEG spectral power and burst-suppression ratio during 0.8% and 1.4% isoflurane anesthesia, respectively. Both male and female mice were used in this study.
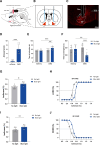
Results: The calcium signals of central amygdala GABAergic neurons decreased during the induction of isoflurane anesthesia and were restored during the emergence. Chemogenetic activation of central amygdala GABAergic neurons delayed induction time (mean ± SD, vehicle vs . clozapine-N-oxide: 58.75 ± 5.42 s vs . 67.63 ± 5.01 s; n = 8; P = 0.0017) and shortened emergence time (385.50 ± 66.26 s vs . 214.60 ± 40.21 s; n = 8; P = 0.0017) from isoflurane anesthesia. Optogenetic activation of central amygdala GABAergic neurons produced a similar effect. Furthermore, optogenetic activation decreased EEG delta power (prestimulation vs . stimulation: 46.63 ± 4.40% vs . 34.16 ± 6.47%; n = 8; P = 0.0195) and burst-suppression ratio (83.39 ± 5.15% vs . 52.60 ± 12.98%; n = 8; P = 0.0003). Moreover, optogenetic stimulation of terminals of central amygdala GABAergic neurons in the basal forebrain also promoted cortical activation and accelerated behavioral emergence from isoflurane anesthesia.
Conclusions: The results suggest that central amygdala GABAergic neurons play a role in general anesthesia regulation, which facilitates behavioral and cortical emergence from isoflurane anesthesia through the GABAergic central amygdala-basal forebrain pathway.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc., on behalf of the American Society of Anesthesiologists.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Wang TX, Xiong B, Xu W, et al. : Activation of parabrachial nucleus glutamatergic neurons accelerates reanimation from sevoflurane anesthesia in mice. Anesthesiology 2019; 130:106–18 - PubMed
-
- Ip CK, Rezitis J, Qi Y, et al. : Critical role of lateral habenula circuits in the control of stress-induced palatable food consumption. Neuron 2023; 111:2583–600.e6 - PubMed
-
- Moscarello JM, Penzo MA: The central nucleus of the amygdala and the construction of defensive modes across the threat-imminence continuum. Nat Neurosci 2022; 25:999–1008 - PubMed
-
- Douglass AM, Kucukdereli H, Ponserre M, et al. : Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat Neurosci 2017; 20:1384–94 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

