STING-dependent induction of neutrophilic asthma exacerbation in response to house dust mite
- PMID: 39466641
- PMCID: PMC11891437
- DOI: 10.1111/all.16369
STING-dependent induction of neutrophilic asthma exacerbation in response to house dust mite
Abstract
Background: Severe refractory, neutrophilic asthma remains an unsolved clinical problem. STING agonists induce a neutrophilic response in the airways, suggesting that STING activation may contribute to the triggering of neutrophilic exacerbations. We aim to determine whether STING-induced neutrophilic lung inflammation mimics severe asthma.
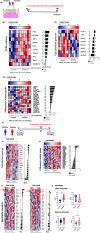
Methods: We developed new models of neutrophilic lung inflammation induced by house dust mite (HDM) plus STING agonists diamidobenzimidazole (diABZI) or cGAMP in wild-type, and conditional-STING-deficient mice. We measured DNA damage, cell death, NETs, cGAS/STING pathway activation by immunoblots, N1/N2 balance by flow cytometry, lung function by plethysmography, and Th1/Th2 cytokines by multiplex. We evaluated diABZI effects on human airway epithelial cells from healthy or patients with asthma, and validated the results by transcriptomic analyses of rhinovirus infected healthy controls vs patients with asthma.
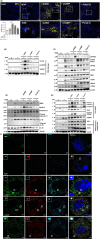
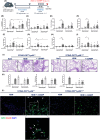
Results: DiABZI administration during HDM challenge increased airway hyperresponsiveness, neutrophil recruitment with prominent NOS2+ARG1- type 1 neutrophils, protein extravasation, cell death by PANoptosis, NETs formation, extracellular dsDNA release, DNA sensors activation, IFNγ, IL-6 and CXCL10 release. Functionally, STING agonists exacerbated airway hyperresponsiveness. DiABZI caused DNA and epithelial barrier damage, STING pathway activation in human airway epithelial cells exposed to HDM, in line with DNA-sensing and PANoptosis pathways upregulation and tight-junction downregulation induced by rhinovirus challenge in patients with asthma.
Conclusions: Our study identifies that triggering STING in the context of asthma induces cell death by PANoptosis, fueling the flame of inflammation through a mixed Th1/Th2 immune response recapitulating the features of severe asthma with a prognostic signature of type 1 neutrophils.
Keywords: DNA sensing; asthma exacerbation; cGAMP; cell death; diABZI; with inserts.
© 2024 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
M.S. reports research grants from Swiss National Science Foundation (nr 310030_189334/1), Novartis Foundation for Medical‐Biological Research, GSK, and Stiftung vorm. Buendner Heilstaette Arosa; speaker's fee from AstraZeneca; voluntary positions in the European Academy of Allergy and Clinical Immunology (EAACI) as Executive Board member and Basic and Clinical Immunology Section Chair. S.L.J. reports grants/contracts from European Research Council ERC FP7 grant number 233015, Chair from Asthma UK CH11SJ, Medical Research Council Centre grant number G1000758, NIHR Biomedical Research Centre grant number P26095, Predicta FP7 Collaborative Project grant number 260895, NIHR Emeritus NIHR Senior Investigator; consulting fees from Lallemand Pharma, Bioforce, resTORbio, Gerson Lehrman Group, Boehringer Ingelheim, Novartis, Bayer, Myelo Therapeutics GmbH; patents issued/licensed: Wark PA, Johnston SL, Holgate ST, Davies DE. Anti‐virus therapy for respiratory diseases. UK patent application No. GB 0405634.7, 12March 2004. Wark PA, Johnston SL, Holgate ST, Davies DE. Interferon‐Beta for Anti‐Virus Therapy for Respiratory Diseases. International Patent Application No. PCT/GB05/50031, 12 March 2004. Davies DE, Wark PA, Holgate ST, Johnston SL. Interferon Lambda therapy for the treatment of respiratory disease. UK patent application No. 6779645.9, granted15th August 2012; Participation on a data safety monitory board or advisory board: Enanta Chair of DSMB, Virtus Respiratory Research Ltd. Shareholder and Board membership. All other authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

