Sequencing of therapy for patients with diffuse large B-cell lymphoma in the era of novel drugs
- PMID: 39466716
- PMCID: PMC11637731
- DOI: 10.1111/bjh.19860
Sequencing of therapy for patients with diffuse large B-cell lymphoma in the era of novel drugs
Abstract
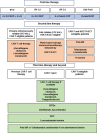
Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive lymphoma, accounting for ~40% of all cases in adults. Whilst approximately two-thirds of DLBCL patients can be cured by first-line therapy, one-third of patients are primary refractory or relapse after an initial response (r/r DLBCL). Recent advances in the treatment of DLBCL have been achieved by a plethora of novel drugs, such as monoclonal antibodies, antibody-drug conjugates (ADC), bi-specific T-cell engagers (BITEs), and CD-19 directed chimeric antigen receptor (CAR)-T-cell therapies. The increasing number of therapeutic options significantly improved the outcome of patients; however, the therapeutic algorithm has become increasingly complex. In this review, we provide an overview of novel therapies for DLBCL patients and potential treatment sequencing from first to second, third, and later lines.
Keywords: DLBCL; antibody‐drug conjugate (ADC); bi‐specific T‐cell engager (BITE); chimeric antigen receptor (CAR)‐T‐cell therapy; high‐dose chemotherapy/autologous stem cell transplantation (HDCT/ASCT); novel drugs; sequencing therapies.
© 2024 The Author(s). British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
I.E.K. is supported by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—493 624 047 (Clinician Scientist CareerS Münster). E.S. received honoraria from Amgen, BMS, Gilead, Lilly, Stemline, Sanofi, Incyte, Takeda, Pfizer and Oncopeptides. N.S. received research grants from Janssen and AstraZeneca. N.S. received honoraria from Roche and travel grants from Beigene holds BMS stocks. G.L. received research grants from AGIOS, AQUINOX, AstraZeneca, Bayer, Celgene, Gilead, Janssen, Morphosys, Novartis, Roche, and Verastem. G.L. received honoraria from ADC Therapeutics, Abbvie, Amgen, AstraZeneca, Bayer, Beigene, BMS, Celgene, Constellation, Genase, Genmab, Gilead, Hexal/Sandoz, Immagene, Incyte, Janssen, Karyopharm, Lilly, Miltenyi, Morphosys, MSD, NanoString, Novartis, PentixaPharm, Pierre Fabre Pharma GmbH, Roche, Sobi, and Takeda.
Figures
References
-
- Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, de Oliveira Araujo IB, Berti E, et al. Correction: “The 5th edition of The World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms”. Leukemia. 2022 Jul;36(7):1720–1748. Leukemia. 2023;37(9):1944–1951. - PMC - PubMed
-
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B‐cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. - PubMed
-
- Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large‐B‐cell lymphoma. N Engl J Med. 2002;346(25):1937–1947. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

