Novel dual-pathogen multi-epitope mRNA vaccine development for Brucella melitensis and Mycobacterium tuberculosis in silico approach
- PMID: 39466745
- PMCID: PMC11515988
- DOI: 10.1371/journal.pone.0309560
Novel dual-pathogen multi-epitope mRNA vaccine development for Brucella melitensis and Mycobacterium tuberculosis in silico approach
Abstract
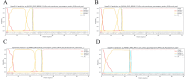
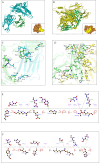
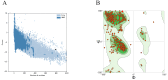
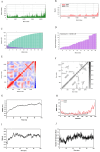
Brucellosis and Tuberculosis, both of which are contagious diseases, have presented significant challenges to global public health security in recent years. Delayed treatment can exacerbate the conditions, jeopardizing patient lives. Currently, no vaccine has been approved to prevent these two diseases simultaneously. In contrast to traditional vaccines, mRNA vaccines offer advantages such as high efficacy, rapid development, and low cost, and their applications are gradually expanding. This study aims to develop multi-epitope mRNA vaccines argeting Brucella melitensis and Mycobacterium tuberculosis H37Rv (L4 strain) utilizing immunoinformatics approaches. The proteins Omp25, Omp31, MPT70, and MPT83 from the specified bacteria were selected to identify the predominant T- and B-cell epitopes for immunological analysis. Following a comprehensive evaluation, a vaccine was developed using helper T lymphocyte epitopes, cytotoxic T lymphocyte epitopes, linear B-cell epitopes, and conformational B-cell epitopes. It has been demonstrated that multi-epitope mRNA vaccines exhibit increased antigenicity, non-allergenicity, solubility, and high stability. The findings from molecular docking and molecular dynamics simulation revealed a robust and enduring binding affinity between multi-epitope peptides mRNA vaccines and TLR4. Ultimately, Subsequently, following the optimization of the nucleotide sequence, the codon adaptation index was calculated to be 1.0, along with an average GC content of 54.01%. This indicates that the multi-epitope mRNA vaccines exhibit potential for efficient expression within the Escherichia coli(E. coli) host. Analysis through immune modeling indicates that following administration of the vaccine, there may be variation in immunecell populations associated with both innate and adaptive immune reactions. These types encompass helper T lymphocytes (HTL), cytotoxic T lymphocytes (CTL), regulatory T lymphocytes, natural killer cells, dendritic cells and various immune cell subsets. In summary, the results suggest that the newly created multi-epitope mRNA vaccine exhibits favorable attributes, offering novel insights and a conceptual foundation for potential progress in vaccine development.
Copyright: © 2024 Zhu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
NO authors have competing interests.
Figures








Similar articles
-
Development of multi-epitope based subunit vaccine against Mycobacterium Tuberculosis using immunoinformatics approach.J Biomol Struct Dyn. 2024;42(22):12365-12384. doi: 10.1080/07391102.2023.2270065. Epub 2023 Oct 25. J Biomol Struct Dyn. 2024. PMID: 37880982
-
A multi-epitope subunit vaccine based on CU/ZN-SOD, OMP31 and BP26 against Brucella melitensis infection in BALB/C mice.Int Immunopharmacol. 2024 Jan 25;127:111351. doi: 10.1016/j.intimp.2023.111351. Epub 2023 Dec 19. Int Immunopharmacol. 2024. PMID: 38113688
-
Designing of a Chimeric Vaccine Using EIS (Rv2416c) Protein Against Mycobacterium tuberculosis H37Rv: an Immunoinformatics Approach.Appl Biochem Biotechnol. 2022 Jan;194(1):187-214. doi: 10.1007/s12010-021-03760-0. Epub 2021 Nov 24. Appl Biochem Biotechnol. 2022. PMID: 34817805
-
Multi-epitopevaccines, from design to expression; an in silico approach.Hum Immunol. 2024 May;85(3):110804. doi: 10.1016/j.humimm.2024.110804. Epub 2024 Apr 23. Hum Immunol. 2024. PMID: 38658216 Review.
-
RNA vaccines: The dawn of a new age for tuberculosis?Hum Vaccin Immunother. 2025 Dec;21(1):2469333. doi: 10.1080/21645515.2025.2469333. Epub 2025 Feb 27. Hum Vaccin Immunother. 2025. PMID: 40013818 Free PMC article. Review.
Cited by
-
Melioidosis vaccines: recent advances and future directions.Front Immunol. 2025 Jun 24;16:1582113. doi: 10.3389/fimmu.2025.1582113. eCollection 2025. Front Immunol. 2025. PMID: 40630947 Free PMC article. Review.
-
Technological breakthroughs and advancements in the application of mRNA vaccines: a comprehensive exploration and future prospects.Front Immunol. 2025 Mar 4;16:1524317. doi: 10.3389/fimmu.2025.1524317. eCollection 2025. Front Immunol. 2025. PMID: 40103818 Free PMC article. Review.
References
-
- Paul S, Peddayelachagiri BV, Gogoi M, Nagaraj S, Ramlal S, Konduru B, Batra HV. Genome-wide unique insertion sequences among five Brucella species and demonstration of differential identification of Brucella by multiplex PCR assay. Sci Rep. 2020;10(1):6368. doi: 10.1038/s41598-020-62472-3 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

